Rep:Mod:captainmurphy
Cope Rearrangement
Optimisation of Reactants and Products
An optimisation calculation of 1,5-Hexadiene with the central carbon atoms in 2-app conformation was run using the HF method and 3-12g basis set.
Item Value Threshold Converged? Maximum Force 0.000060 0.000450 YES RMS Force 0.000010 0.000300 YES Maximum Displacement 0.000525 0.001800 YES RMS Displacement 0.000171 0.001200 YES Predicted change in Energy=-2.037394D-08 Optimization completed. -- Stationary point found.
The calculation had the following results
Hex-1,5-diene optimisation File Name HEX15DIENE_OPTIM File Type .log Calculation Type FOPT Calculation Method RHF Basis Set 3-21G Charge 0 Spin Singlet E(RHF) -231.69253528 a.u. RMS Gradient Norm 0.00001891 a.u. Imaginary Freq Dipole Moment 0.0000 Debye Point Group C1
A minimum was converged upon. Ideally, the conformation should have a Ci point group, however, due to slight differences between each bond length and bond angle, the optimised molecule has no centre of inversion there a C1 point group. Link to the .log file below
https://wiki.ch.ic.ac.uk/wiki/images/d/dd/HEX15DIENE_OPTIM.LOG
A optimisation calculation of lower energy antiperiplanar conformer, app1, of 1,5-hexadiene was run using the HF method and 3-12g basis set.
Item Value Threshold Converged? Maximum Force 0.000020 0.000450 YES RMS Force 0.000007 0.000300 YES Maximum Displacement 0.000668 0.001800 YES RMS Displacement 0.000201 0.001200 YES Predicted change in Energy=-1.761864D-08 Optimization completed. -- Stationary point found.
The calculation had the following results:
1app 1,5-hexadiene optimisation File Name app1_15hexadiene_optim_output File Type .log Calculation Type FOPT Calculation Method RHF Basis Set 3-21G Charge 0 Spin Singlet E(RHF) -231.69260236 a.u. RMS Gradient Norm 0.00001217 a.u. Imaginary Freq Dipole Moment 0.2023 Debye Point Group C1
The calculation was published on D-space: DOI:10042/24099
An optimisation calculation of the gauche 3 conformation of 1,5-Hexadiene was run using the HF method and 3-12g basis. The optimised molecule also had a C1 point group, though ideally it should have C2 point group
Item Value Threshold Converged? Maximum Force 0.000014 0.000450 YES RMS Force 0.000003 0.000300 YES Maximum Displacement 0.000498 0.001800 YES RMS Displacement 0.000192 0.001200 YES Predicted change in Energy=-4.041803D-09 Optimization completed. -- Stationary point found.
The Calculation had the following results
Gauche3 1,5-Hexadiene optimisation File Name gauche3_15hexadiene_optim_output File Type .log Calculation Type FOPT Calculation Method RHF Basis Set 3-21G Charge 0 Spin Singlet E(RHF) -231.69266122 a.u. RMS Gradient Norm 0.00000446 a.u. Imaginary Freq Dipole Moment 0.3407 Debye Point Group C1
The calculation was published on D-space DOI:10042/24075
The app2 conformer was re-optimised using the DFT method and 6-31G basis set.
Item Value Threshold Converged? Maximum Force 0.000015 0.000450 YES RMS Force 0.000006 0.000300 YES Maximum Displacement 0.000219 0.001800 YES RMS Displacement 0.000079 0.001200 YES Predicted change in Energy=-1.589047D-08 Optimization completed. -- Stationary point found.
The results for the calculations are as follows:
Hex-1,5-diene optimisation File Name app2_15hexadiene_optim_DFT_output File Type .log Calculation Type FOPT Calculation Method RB3LYP Basis Set 6-31G(d) Charge 0 Spin Singlet E(RB3LYP) -234.61170280 a.u. RMS Gradient Norm 0.00001326 a.u. Imaginary Freq Dipole Moment 0.0000 Debye Point Group CI
The calculation was published on D-space: DOI:10042/24115
Intriguingly, the point group of the reoptimised molecule is Ci, thus the centre of inversion has been restored
A frequency calculation was then run of the reoptimised molecule using the same method and basis set. The calculation was published on D-space: DOI:10042/24147
The following sums of energies were taken a note of:
Sum of electronic and zero-point Energies= -234.469212 Sum of electronic and thermal Energies= -234.461856 Sum of electronic and thermal Enthalpies= -234.460912 Sum of electronic and thermal Free Energies= -234.500821
Optimisation of "Chair" and "Boat" Transition Structures
An allyl fragment was drawn and optimised to HF/3-21g level of theory.
Item Value Threshold Converged? Maximum Force 0.000044 0.000450 YES RMS Force 0.000018 0.000300 YES Maximum Displacement 0.000175 0.001800 YES RMS Displacement 0.000073 0.001200 YES Predicted change in Energy=-1.235912D-08 Optimization completed. -- Stationary point found.
The calculation was published on D-space: DOI:10042/24161
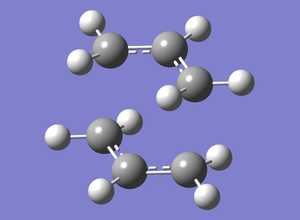
A guess transition state was drawn using two allyl fragments with a proximity of roughly 2.2Å to each other(2.20688 and 2.20198Å).
This was first optimised in a single optimisation and frequency calculation. The two fragments were optimised to a transition state with force constants calculated once. opt=noeigen was used as an additional keyword. The calculation was run to HF/3-21g level of theory.
Item Value Threshold Converged? Maximum Force 0.000009 0.000450 YES RMS Force 0.000003 0.000300 YES Maximum Displacement 0.000216 0.001800 YES RMS Displacement 0.000061 0.001200 YES Predicted change in Energy=-6.255335D-09 Optimization completed. -- Stationary point found.
An imaginary frequency of about 818cm-1 was calculated (N.B. that it has a negative sign in the log file)
Low frequencies --- -817.9412 -1.7066 -1.2826 0.0006 0.0007 0.0009 Low frequencies --- 1.9264 209.5416 396.0238
The two ends were 2.02036 and 2.02042Å apart from each other, so there had been a change of the fragments' positions.
The calculation was published on D-space: DOI:10042/24163

The guess structure was optimised again, however this time just in a single optimisation calculation. Coordinates were set for each of the carbon atom bond breaking/forming pairs. Both of the coordinates were set as bond; freeze coordinates. The structure was optimised to a minimum with force constants not calculated. Again, the calculation was run to HF/3-21g level of theory.
Item Value Threshold Converged? Maximum Force 0.000034 0.000450 YES RMS Force 0.000012 0.000300 YES Maximum Displacement 0.001212 0.001800 YES RMS Displacement 0.000369 0.001200 YES Predicted change in Energy=-1.814707D-07 Optimization completed. -- Stationary point found.
The distance between the fragment ends were 2.20688 and 2.20198Å, i.e. the position of the fragments had not changed.
The calculation was published on D-space: DOI:10042/24165
This structure was then reoptimised, however, the coordinates were adjusted to bond; derivative of each pair and the structure was optimised to a transition state. HF/3-21g level of theory was used.
Item Value Threshold Converged? Maximum Force 0.000049 0.000450 YES RMS Force 0.000013 0.000300 YES Maximum Displacement 0.001381 0.001800 YES RMS Displacement 0.000273 0.001200 YES Predicted change in Energy=-6.950108D-07 Optimization completed. -- Stationary point found.
The distance between the fragment ends were 2.01913 and 2.01910Å.
The calculation was published on D-space: DOI:10042/24169

Considering the energy of app2 1,5-Hexadiene and the chair transition state, the activation energy is about 45.8Kcalmol-1, which significantly higher than the literature2 value of 34Kcalmol-1, thus the transition state is likely to be in a boat conformation.
Diels-Alder
Butadiene
A cis-butadiene molecule was optimised to the semi-empirical AM1 level of theory. The calculation converged upon a single point.
Item Value Threshold Converged? Maximum Force 0.000025 0.000450 YES RMS Force 0.000011 0.000300 YES Maximum Displacement 0.000318 0.001800 YES RMS Displacement 0.000128 0.001200 YES Predicted change in Energy=-8.152977D-09 Optimization completed. -- Stationary point found.
The optimised molecule has Cs point group symmetry. This is caused by a sigma symmetry plane across the molecule.
Link to the calculation's log file:
https://wiki.ch.ic.ac.uk/wiki/images/6/6e/CIS_BUTADIENE_OPTIM.LOG
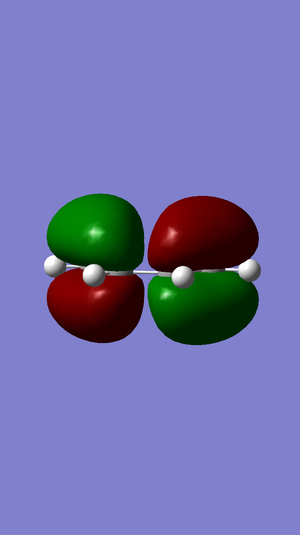
A population calculation of the molecule was run and HOMO and LUMO was visualised. The HOMO has a A' symmetry where as the LUMO has a A symmetry.
| HOMO | LUMO |
|---|---|
 |
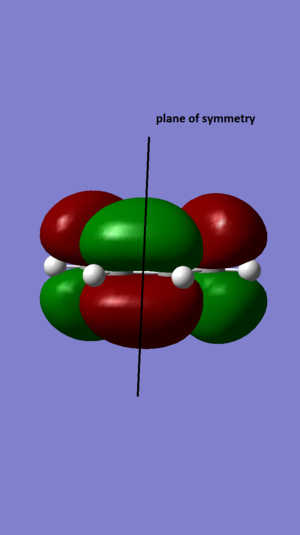 |
| Antisymmetric in the plane | Antisymmetric in the plane |
Link to the calculation's log file:
https://wiki.ch.ic.ac.uk/wiki/images/6/61/CIS_BUTADIENE_MO.LOG
A frequency calculation was run, revealing that the optimised planar cis butadiene in fact is a transition state. This is indicated by the imaginary (negative) frequency.
Low frequencies --- -39.9802 -5.7100 -3.4687 -2.2322 -0.0003 0.0149
Low frequencies --- 0.0469 312.4533 485.1744
****** 1 imaginary frequencies (negative Signs) ******
Harmonic frequencies (cm**-1), IR intensities (KM/Mole), Raman scattering
activities (A**4/AMU), depolarization ratios for plane and unpolarized
incident light, reduced masses (AMU), force constants (mDyne/A),
and normal coordinates:
1 2 3
A" A' A"
Frequencies -- -39.9801 312.4533 485.1744
Red. masses -- 1.4869 2.6016 1.1394
Frc consts -- 0.0014 0.1496 0.1580
IR Inten -- 0.0000 0.0339 7.9405
Link to the calculation's log file: https://wiki.ch.ic.ac.uk/wiki/images/b/b8/CIS_BUTADIENE_FREQ.LOG
Another cis-butadiene molecule was drawn with a dihedral twist between the two central carbons. This was optmised and frequencies were calculated in single operation carried out to the semi-empirical AM1 level of theory.
Item Value Threshold Converged? Maximum Force 0.000027 0.000450 YES RMS Force 0.000009 0.000300 YES Maximum Displacement 0.001345 0.001800 YES RMS Displacement 0.000497 0.001200 YES Predicted change in Energy=-9.201512D-09 Optimization completed. -- Stationary point found.
The calculation had converged on a single point.
The frequencies were all positive, thus indicating that this is a possible conformation of cis-butadiene.
Low frequencies --- -3.1387 -2.6778 -1.6416 0.0032 0.1230 0.2774
Low frequencies --- 53.8133 304.3830 480.2925
Harmonic frequencies (cm**-1), IR intensities (KM/Mole), Raman scattering
activities (A**4/AMU), depolarization ratios for plane and unpolarized
incident light, reduced masses (AMU), force constants (mDyne/A),
and normal coordinates:
1 2 3
A A A
Frequencies -- 53.8126 304.3830 480.2925
Red. masses -- 1.4974 2.5655 1.1704
Frc consts -- 0.0026 0.1400 0.1591
IR Inten -- 0.0251 0.0412 7.6793
The calculation was published on D-space DOI:10042/24239
Transition State of Diels-Alder Reaction
A guess structure of the transition state was drawn and then optimised to a transition state in a joint optimisation/frequency calculation to the HF/3-21g level of theory.
Item Value Threshold Converged? Maximum Force 0.000057 0.000450 YES RMS Force 0.000009 0.000300 YES Maximum Displacement 0.001130 0.001800 YES RMS Displacement 0.000262 0.001200 YES Predicted change in Energy=-1.850229D-08 Optimization completed. -- Stationary point found.
The fact that is was a transition state was confirmed as there were imaginary frequencies calculated
Low frequencies --- -818.1909 -2.6568 -1.9920 -0.0007 -0.0007 -0.0007
Low frequencies --- 1.1139 166.6501 284.3579
****** 1 imaginary frequencies (negative Signs) ******
Harmonic frequencies (cm**-1), IR intensities (KM/Mole), Raman scattering
activities (A**4/AMU), depolarization ratios for plane and unpolarized
incident light, reduced masses (AMU), force constants (mDyne/A),
and normal coordinates:
1 2 3
A A A
Frequencies -- -818.1909 166.6500 284.3578
Red. masses -- 7.0080 2.0105 4.4032
Frc consts -- 2.7641 0.0329 0.2098
IR Inten -- 9.3180 0.6930 1.1452
Raman Activ -- 186.0085 0.1520 5.9205
Depolar (P) -- 0.4421 0.7500 0.7500
Depolar (U) -- 0.6131 0.8571 0.8571
The imaginary frequency being the negative one.
The transition state is of the Cs point group, thanks to having a σ plane of symmetry.
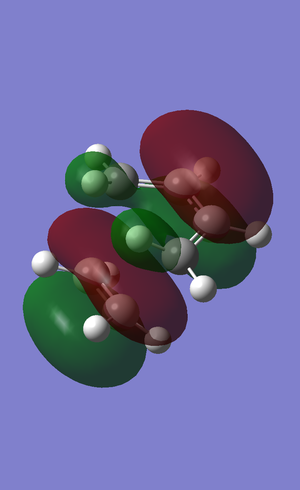
A population calculation was run the HOMO and LUMO of the transition state were visualised
| HOMO | LUMO |
|---|---|
 |
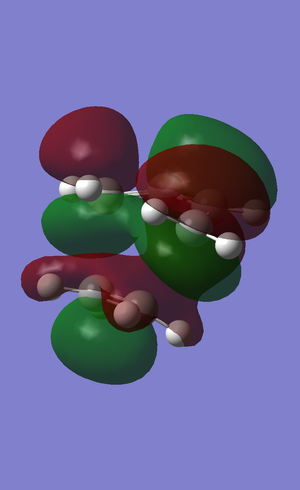 |
The HOMO of the transition state consists of a somewhat distorted pi bonding MO of ethene and a pi-bonding MO within the diene, however, the bottom lobe extends over the terminal carbon atoms of the diene. There is a node, therefore antibonding interaction, between the ethene's MO and the diene's MO.
The LUMO of the transition state consists of a MO of the diene that resembles the LUMO of the isolated diene, however, here a two lobes that are beneath the two terminal carbons of the diene join up as a single lobe. There is an MO that covers the ethene fragment which again resembles a distorted pi-bonding MO, however, here the ethene MO is more distorted as the upper lobe extends up to the diene fragment and there is a strong bonding interaction with the central bottom lobe.
The transition state was reoptimised again in a joint optimistaion/frequency calculation to the DFT/6-31g* level of theory.
Item Value Threshold Converged? Maximum Force 0.000041 0.000450 YES RMS Force 0.000012 0.000300 YES Maximum Displacement 0.001237 0.001800 YES RMS Displacement 0.000362 0.001200 YES Predicted change in Energy=-7.523562D-08 Optimization completed. -- Stationary point found.
Again, the fact that this was a transition state was confirmed by the presence of an imaginary frequency in the frequency analysis
Low frequencies --- -524.7891 -8.0596 -0.0007 -0.0004 -0.0001 9.1064
Low frequencies --- 19.3303 135.8146 203.8497
****** 1 imaginary frequencies (negative Signs) ******
Harmonic frequencies (cm**-1), IR intensities (KM/Mole), Raman scattering
activities (A**4/AMU), depolarization ratios for plane and unpolarized
incident light, reduced masses (AMU), force constants (mDyne/A),
and normal coordinates:
1 2 3
A A A
Frequencies -- -524.7888 135.7607 203.8373
Red. masses -- 8.2420 2.1652 3.9546
Frc consts -- 1.3374 0.0235 0.0968
IR Inten -- 5.8120 0.7227 0.9943
Regioselectivity when Maleic Anhydride is used as the Dienophile
For the Diels-Alder reaction between cyclohexadiene and maleic anhydride, there are two isomers that can be the product: the endo adduct and the exo adduct. One of these adducts is the predominant product when the reaction is under kinetic control, whereas the other is thermodynamic product.
A guess structure of cyclohexadiene was drawn and optimised to the semi-empirical/AM1 level of theory in a joint optimisation and frequency calculation.
Item Value Threshold Converged? Maximum Force 0.000079 0.000450 YES RMS Force 0.000015 0.000300 YES Maximum Displacement 0.000811 0.001800 YES RMS Displacement 0.000243 0.001200 YES Predicted change in Energy=-4.350086D-08 Optimization completed. -- Stationary point found.
The frequencies confirmed that the optimised structure was realistic, i.e. all the frequencies were positive
Low frequencies --- -4.6495 -1.1995 -0.0160 0.0590 0.1371 4.3120
Low frequencies --- 99.3786 276.7750 457.6047
Harmonic frequencies (cm**-1), IR intensities (KM/Mole), Raman scattering
activities (A**4/AMU), depolarization ratios for plane and unpolarized
incident light, reduced masses (AMU), force constants (mDyne/A),
and normal coordinates:
1 2 3
A A A
Frequencies -- 99.3784 276.7750 457.6047
Red. masses -- 1.6772 2.0820 1.9360
Frc consts -- 0.0098 0.0940 0.2389
IR Inten -- 0.0712 0.0870 0.0075
The diene has a total energy of 0.02771132 Hartrees
Link to the calculation's .log file: https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:CYCLOHEXADIENE_OPTIM_FREQ.LOG
A guess structure of Maleic anhydride was drawn and optimised to the semi-empirical/AM1 level of theory in a joint optimisation - frequency calculation.
Item Value Threshold Converged? Maximum Force 0.000167 0.000450 YES RMS Force 0.000056 0.000300 YES Maximum Displacement 0.000907 0.001800 YES RMS Displacement 0.000312 0.001200 YES Predicted change in Energy=-1.502697D-07 Optimization completed. -- Stationary point found.
The frequencies confirmed that the optimised structure was realistic, i.e. all the frequencies were positive
Low frequencies --- -0.0180 -0.0104 -0.0008 2.8733 6.4107 11.3007
Low frequencies --- 155.8845 265.8974 382.8494
Harmonic frequencies (cm**-1), IR intensities (KM/Mole), Raman scattering
activities (A**4/AMU), depolarization ratios for plane and unpolarized
incident light, reduced masses (AMU), force constants (mDyne/A),
and normal coordinates:
1 2 3
A A A
Frequencies -- 155.8845 265.8973 382.8494
Red. masses -- 15.8572 3.6760 13.5036
Frc consts -- 0.2270 0.1531 1.1662
IR Inten -- 1.0503 0.0000 23.8302
The anhydride has a total energy of -0.12182409 Hartrees
Link to the calculation's .log file: https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:MALEIC_ANHYDRIDE_OPTIM_FREQ.LOG
A guess structure of endo-adduct was drawn and optimised to the semi-empirical/AM1 level of theory in a joint optimisation - frequency calculation.
Item Value Threshold Converged? Maximum Force 0.000102 0.000450 YES RMS Force 0.000012 0.000300 YES Maximum Displacement 0.000840 0.001800 YES RMS Displacement 0.000189 0.001200 YES Predicted change in Energy=-3.874703D-08 Optimization completed. -- Stationary point found.
The frequencies confirmed that the optimised structure was realistic, i.e. all the frequencies were positive
Low frequencies --- -2.6633 -0.6840 -0.2503 -0.0034 0.1130 1.7882
Low frequencies --- 72.1977 148.2059 167.4255
Harmonic frequencies (cm**-1), IR intensities (KM/Mole), Raman scattering
activities (A**4/AMU), depolarization ratios for plane and unpolarized
incident light, reduced masses (AMU), force constants (mDyne/A),
and normal coordinates:
1 2 3
A A A
Frequencies -- 72.1976 148.2059 167.4255
Red. masses -- 5.1640 9.0542 7.5959
Frc consts -- 0.0159 0.1172 0.1255
IR Inten -- 0.1402 4.9418 0.9083
The adduct has a total energy of -0.16017081 Hartrees
Link to the calculation's .log file: https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:ENDO_ADDUCT_OPTIM_FREQ.LOG
A guess structure of exo-adduct was drawn and optimised to the semi-empirical/AM1 level of theory in a joint optimisation - frequency calculation.
Item Value Threshold Converged? Maximum Force 0.000060 0.000450 YES RMS Force 0.000012 0.000300 YES Maximum Displacement 0.000656 0.001800 YES RMS Displacement 0.000142 0.001200 YES Predicted change in Energy=-1.026530D-07 Optimization completed. -- Stationary point found.
The frequencies confirmed that the optimised structure was realistic, i.e. all the frequencies were positive
Low frequencies --- -2.6160 -2.2275 -0.2565 -0.0027 0.1138 1.5370
Low frequencies --- 70.6655 148.5658 168.7536
Harmonic frequencies (cm**-1), IR intensities (KM/Mole), Raman scattering
activities (A**4/AMU), depolarization ratios for plane and unpolarized
incident light, reduced masses (AMU), force constants (mDyne/A),
and normal coordinates:
1 2 3
A A A
Frequencies -- 70.6654 148.5658 168.7536
Red. masses -- 5.1773 11.0120 6.0630
Frc consts -- 0.0152 0.1432 0.1017
IR Inten -- 0.1457 4.1071 1.9500
The adduct has a total energy of -0.15990931 Hartrees, therefore the endo adduct is the more thermodynamically stable out of the two products.
Link to the calculation's .log file: https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:EXO_ADDUCT_OPTIM_FREQ.LOG
The optimised reactants and products were used to attain the transition states for the two possible paths of the reaction.
Firstly the endo adduct was used as the product with cyclohexadiene and maleic anhydride as the reactants. A joint optimisation-frequency calculation was set up, optimising to QST2 transition state at the semi-empirical/AM1 level of theory.
Item Value Threshold Converged? Maximum Force 0.000017 0.000450 YES RMS Force 0.000002 0.000300 YES Maximum Displacement 0.000197 0.001800 YES RMS Displacement 0.000039 0.001200 YES Predicted change in Energy=-2.144656D-09 Optimization completed. -- Stationary point found.
The frequencies confirmed that a transition state had been converged on as there was an imaginary (negative frequency) which was caused by the bond forming vibrations.
Low frequencies --- -806.3608 -1.1611 -1.0189 -0.2526 -0.0104 0.7037
Low frequencies --- 1.7526 62.4360 111.7370
****** 1 imaginary frequencies (negative Signs) ******
Harmonic frequencies (cm**-1), IR intensities (KM/Mole), Raman scattering
activities (A**4/AMU), depolarization ratios for plane and unpolarized
incident light, reduced masses (AMU), force constants (mDyne/A),
and normal coordinates:
1 2 3
A A A
Frequencies -- -806.3608 62.4360 111.7370
Red. masses -- 6.7020 4.3325 6.8014
Frc consts -- 2.5675 0.0100 0.0500
IR Inten -- 71.6009 1.5331 3.4385
The transition state has a total energy of -0.05150480 Hartrees
Link to the calculation's .log file:
https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:ENDO_ADDUCT_TS_OPTIM_FREQ.LOG

The exo adduct transition state was found using a similar method. With the exo adduct assigned as the product and the cyclohexadiene and maleic anhydride as the reactants, a joint optimisation-frequency calculation was set up to converge on a QST2 transition state to the semi-empirical/AM1 level of theory.
Item Value Threshold Converged? Maximum Force 0.000019 0.000450 YES RMS Force 0.000002 0.000300 YES Maximum Displacement 0.000905 0.001800 YES RMS Displacement 0.000219 0.001200 YES Predicted change in Energy=-9.745176D-09 Optimization completed. -- Stationary point found.
Again the frequencies confirmed that a transition state had been converged upon as there was an imaginary (negative) frequency.
Low frequencies --- -812.2048 -1.3419 -0.9671 -0.0047 0.3850 1.0398
Low frequencies --- 2.2924 60.8664 123.8706
****** 1 imaginary frequencies (negative Signs) ******
Harmonic frequencies (cm**-1), IR intensities (KM/Mole), Raman scattering
activities (A**4/AMU), depolarization ratios for plane and unpolarized
incident light, reduced masses (AMU), force constants (mDyne/A),
and normal coordinates:
1 2 3
A A A
Frequencies -- -812.2048 60.8664 123.8706
Red. masses -- 7.0435 4.4895 7.1640
Frc consts -- 2.7376 0.0098 0.0648
IR Inten -- 96.8769 0.5530 0.0413
The transition state has a total energy of -0.05041984 Hartrees
Link to the calculation's .log file:
https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:EXO_ADDUCT_TS_OPTIM_FREQ.LOG
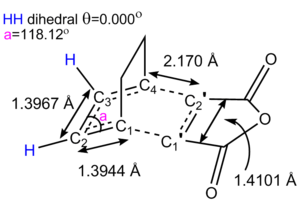
For the formation of the endo adduct, there is an activation energy of 0.04260797 hartrees, whereas the activation energy for the exo adduct is 0.04369293 hartrees, therefore the endo adduct is the thermodynamic and kinetic product.
There was a change of distances between many of the atoms due to the making and breaking of several carbon carbon bonds.
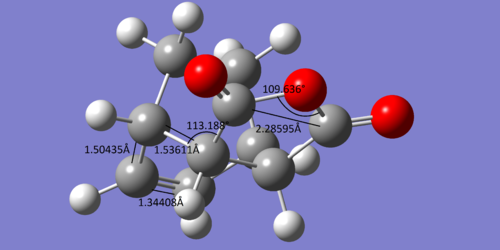
For the endo structures, the bond lengths of interest are as follows:
| Transition State | Adduct | ||
|---|---|---|---|
| cell | 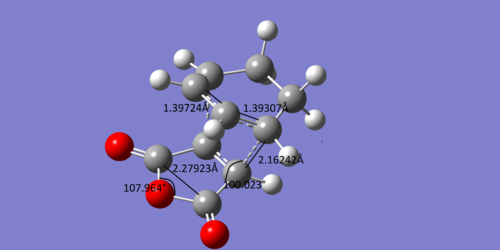 |
 |
cell |
| cell | cell | cell | cell |
The bond lengths of the transition structure more closely resemble those of the reactants than the adduct's bond lengths. Considering that along with the fact the change in entropy of this reaction would be negative as two molecules are forming one, the reaction may be exothermic and may have an early have an early transition state which would more closely resemble the reactants in accordance with Hammond's postulate.
In particular, the carbon carbon double bond lengths in cyclohexadiene are 1.34279Å, which then extends to 1.39307Å in the conjugated transition state, which then finally reaches 1.50265Å in the adduct, by which point the bond is now a single bond between a sp2 and sp3 hybridised carbons, which is in good agreement with literature1 values of 1.50Å for such bonds.
The single bond between the two double bonds in cyclohexadiene is initially 1.44955Å, though this is shortened to 1.39724Å in the transition state and finally 1.34385Å in the adduct, by which point it is a carbon carbon double bond. In both of these circumstances, the transition state bond lengths more similar in length to the reactants than the products.
The angle between C=O-C-H bond and the forming C-C sigma bond is about 100°, where as the equivalent angle for the adduct is about 113°. If it is assumed that the maleic anhydride approaches the diene at 90°, then this suggests that the transition state is just before the halfway point in the reaction coordinate.
There are similar results for the exo adduct and transition state
| Transition State | Adduct | ||
|---|---|---|---|
| cell | 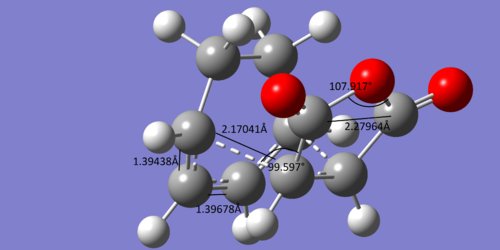 |
 |
cell |
| cell | cell | cell | cell |
The bond lengths again suggest that the transition state occurs early on in the reaction and the angle of the forming bond again suggests that the transition state is just before the halfway point of the reaction.
A population calculation was run on each of the adducts and their transition state.
| Transition State | Adduct | |
|---|---|---|
| LUMO | 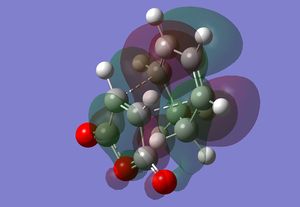 |
 |
| HOMO |  |
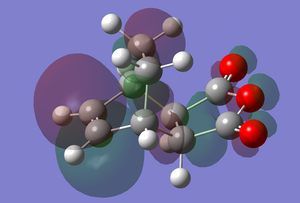 |
The HOMO of the transition state consists of a group of lobes that resemble the HOMO of cis-butadiene, however, here one of the lobes extend over the maleic anhydride fragment, especially where the new sigma bonds are being formed. This lobe shows secondary orbital overlap as both as there contributions from orbitals that are not directly involved in the bond breaking and making[3]. There is a lobe (which is in the same phase as the aforementioned lobe) that extends from the two bottom sp3 hybridised carbons of cyclohexadiene to one of the carbonyl oxygen atoms. That oxygen atom is the nodal point of the lobe, with a lobe of the opposite phase on the other side of the oxygen. There is a similar p-orbital like lobe on the other carbonyl oxygen, however this doesn't interact with other lobes, there is also a similar one on the central oxygen, though this is much smaller. There is a small lobe that is sandwiched in between the two previously mentioned extended lobes, which of the opposite phase. There is also an extended lobe over three carbon atoms of the maleic anhydride fragment.
The LUMO of the transition state is similar in that there is group of lobes that resemble the LUMO of cis-butadiene, however one lobe extends to one of the carbonyl oxygens again, which also displays secondary orbital overlap, and another extends over some of the anhydride fragment.
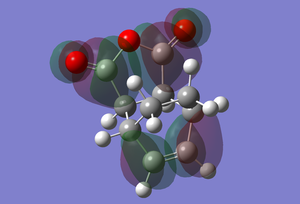
The HOMO and LUMO of the adduct are comparitively localised and a lobe barely extends over more than two atoms at most. The HOMO consists of a distorted pi-bonding lobe over the newly formed carbon carbon double bond in the 'cyclohexadiene' fragment as well as two smaller lobe pairs over the CH2-CH2 fragments in the new bicyclic system. Each oxygen has a p orbital like lobe on it which localised on each atom.
The LUMO largely consists of antibonding interaction lobes. Firstly, there is a pi* antibonding orbital focused on the new carbon carbon double bond that extends onto the adjacent carbons. There are also pi* antibonding orbital over each Carbon Oxygen double bond that also extends over the adjacent carbon atoms.
| Transition State | Adduct | |
|---|---|---|
| LUMO | 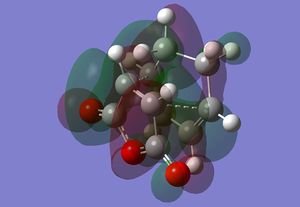 |
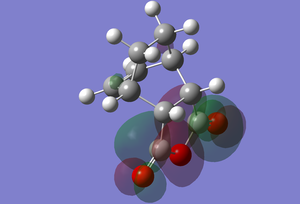 |
| HOMO | 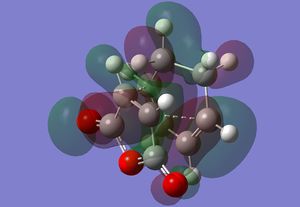 |
 |
The HOMO of the transition state is like the that of the exo transition state in that there is a molecular orbital that resembles the HOMO of cis-butadiene and one of the lobes extends elsewhere throughout the molecule, however, here that lobe is a bit more diffuse and extends over the ring of the anhydride in a spiral like manner, ending with joining up with a p orbital like lobe on the central oxygen. This lobe appears to show secondary orbital overlap. The carbonyl oxygens have localised p orbital like lobes. As for the CH2-CH2 fragment from the cyclohexadiene, there is a lobe pair over a CH2 with the carbon as the nodal point, with one of the lobes extending diagonally onto the next carbon, where it is there paired with a smaller lobe in the opposite phase.
The LUMO mainly consists of highly extended lobes. The main feature is that there is a node where one of the carbon carbon sigma bonds are forming whereas there appears to only be constructive interactions around where the other bond is being formed.
The HOMO for the adduct is very similar in comparison to the exo's equivalent, mainly in that there is a pi bonding interaction over the new carbon carbon double bond and there are similar orbitals about the other CH2-CH2 fragments. Only the central oxygen, however, has a p orbital like lobe about it however.
The LUMO again is similar in that there are pi* antibonding orbitals about the carbon oxygen double bonds, however, there is no antibonding about the carbon carbon double bond. There is instead a small lobe on the adjacent carbons, each lobe being of the opposite phase.
References
1. Fox, Marye Anne; Whitesell, James K. (1995). Organische Chemie: Grundlagen, Mechanismen, Bioorganische Anwendungen. Springer. ISBN 978-3-86025-249-9. 2. https://webspace.utexas.edu/ccl524/CH318N/33-sigma.pdf 3. M. A. Fox, R. Cardona and N. J. Kiwiet, J. Org. Chem. 1987,52, 1469-1474
