Rep:Mod:c01190004
BH3
3_1G, B3LYP Optimisation
Optimised bond length= 1.19467 a.u.
Optimised bond angle = 120.0°
-
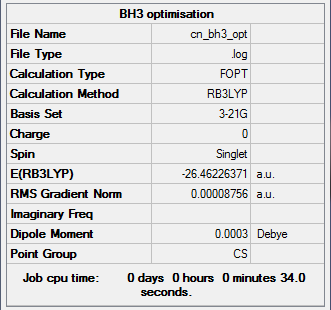
BH3 optimisation summary of results
Optimisation is complete as the gradient norm has reached a low value of 0.00008756 a.u. ( <0.001 a.u. )
Link to log file: File:CN_BH3_OPT.LOG
Extract of Item table from the .log file:
Item Value Threshold Converged?
Maximum Force 0.000217 0.000450 YES
RMS Force 0.000105 0.000300 YES
Maximum Displacement 0.000692 0.001800 YES
RMS Displacement 0.000441 0.001200 YES
Predicted change in Energy=-1.635268D-07
Optimization completed.
-- Stationary point found.
The important information is in the part below "Item" this tells us that the forces are converged (remember force is the gradient or slope of the energy vs distance graph. It also tells us that the placements are converged, this means that for a small displacement the energy does not change
Q: What definition would you choose for the existence of a bond?
The definition i would choose would be that the bond distance is lesser than half of the sum of the Van Der Waal's radii of the two atoms in consideration.
Smf115 (talk) 16:20, 26 May 2018 (BST)Nice to see the 3-21G calculation and the symmetrisation presented and awareness shown towards the optimisation procedure and the associated convergence criteria. The next (additional) step would be to consider why the basis set was improved and why the molecule was symmetrised? This isn't necessary for the report but a good consideration.
6_31G (d,p), B3LYP Optimisation
The output of the 3_31G run was utilised to run a 2nd optimisation using a higher level basis set: 6_31G (d,p)
Running the 6_31g calculation provides a molecule with the the optimised structure as can be intepreted from the low gradient of 0.00008206 a.u. ( <0.001 a.u.)and convergence has occurred as from the Item table in the .log file.
-
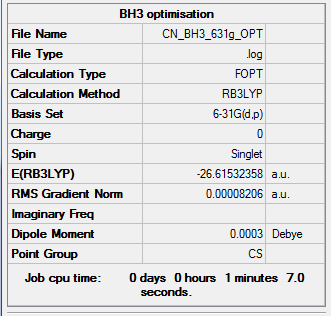
BH3 optimisation (using higher level basis set: 6-31G(d,p))
Link to log fileː File:CN_BH3_631G_OPT.LOG
Extract of Item table from log fileː
Item Value Threshold Converged?
Maximum Force 0.000204 0.000450 YES
RMS Force 0.000099 0.000300 YES
Maximum Displacement 0.000875 0.001800 YES
RMS Displacement 0.000418 0.001200 YES
Predicted change in Energy=-1.452109D-07
Optimization completed.
-- Stationary point found.
We can hence conclude that the run has concluded properly.
Total energy (631g): -26.61532 a.u.
Total energy (31g)= -26.46226 a.u.
Energy difference = 0.15305 a.u.
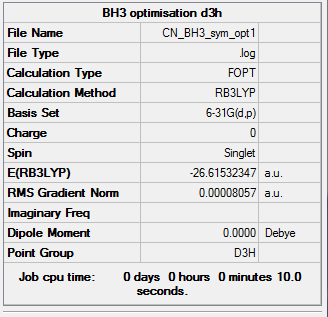
The optimisation was re-run with a D3H symmetry imposed on it.
Following the optimisation run, a D3H symmetry molecule was obtained.
-
BH3 optimisation using 6-31G(d,p), constraining to D3H
Link to log file: File:CN_BH3_SYM_OPT1.LOG
Extract of the Item table ː
Item Value Threshold Converged?
Maximum Force 0.000161 0.000450 YES
RMS Force 0.000105 0.000300 YES
Maximum Displacement 0.000639 0.001800 YES
RMS Displacement 0.000418 0.001200 YES
Predicted change in Energy=-1.545207D-07
Optimization completed.
-- Stationary point found.
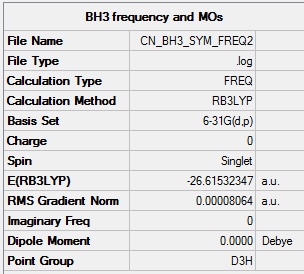
Frequency analysis
A frequency run was done on the output of the BH3 6_31G optimisation run, and the energy is the same as the input at -26.61532 a.u. as seen below.
-
BH3 frequency run using optimisation with 6-31G(d,p) basis set
Link to log fileː File:CN_BH3_SYM_FREQ2.LOG
Low frequencies --- -0.2433 -0.1118 -0.0055 44.4606 45.6068 45.6075 Low frequencies --- 1163.6172 1213.6006 1213.6033
The lowest frequency range is within ±50 cm-1 range, and hence this run is accepted as successful.
Item Value Threshold Converged?
Maximum Force 0.000161 0.000450 YES
RMS Force 0.000081 0.000300 YES
Maximum Displacement 0.000635 0.001800 YES
RMS Displacement 0.000318 0.001200 YES
Predicted change in Energy=-1.536878D-07
Optimization completed.
-- Stationary point found.
The formally zero frequencies are well separated from the lowest energy positive frequency at 1089 cm-1 and the large formally zero frequencies are due to the low level of the basis set and relatively relaxed convergence and integration criteria, which can occur for small molecules like NH3.
Vibrational spectrum for BH3
| Vibrations (cm-1 ) | Intensities(arbitrary) | Symmetry | IR active | Type |
|---|---|---|---|---|
| 1163 | 6.6 | A2" | Yes | Symmetric bend |
| 1213 | 1 | E' | Very slightly | Asymetric bend |
| 1213 | 1 | E' | Very slightly | Asymetric bend |
| 2580 | 0 | A1' | No | Symmetric stretch |
| 2713 | 9 | E' | Yes | Asymmetric stretch |
| 2713 | 9 | E' | Yes | Asymmetric stretch |
Snapshot of the IR spectrum:
-
IR spectrum of BH3
There are only 3 peaks in the spectrum. This is because the 2nd, 3rd vibrations are degenerate, and so are the 4th and 5th vibrations in the table, for within these pairs, the vibrations have the same wavenumber and intensity given that wavenumber is proportional to energy. Because they are degenerate and of the same energy, they give rise to a single peak in the spectra. The fourth vibration is not observed as it has an intensity of 0 and will not be experimentally observable. Hence only three peaks corresponding to the 1st, 2nd, 3rd, 5th, 6th vibrations in the table (of which 2nd, 3rd are degenerate and 5th,6th are degenerate), will be observed.
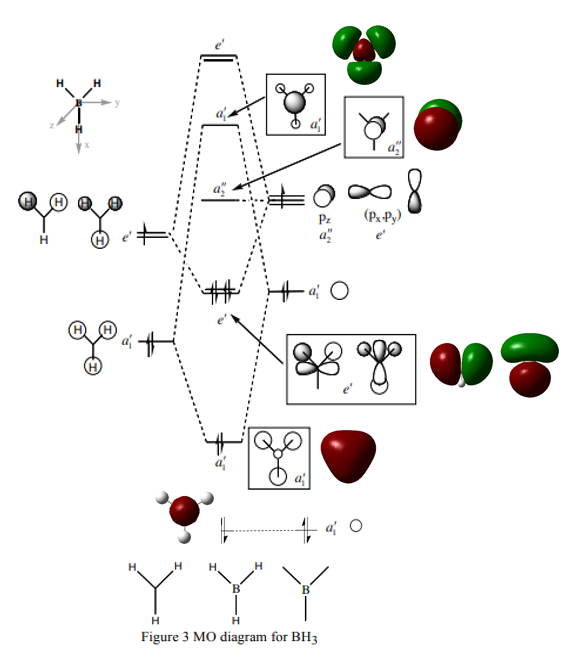
Molecular orbitals of BH3
Modified MO diagram of BH3 with the calculated MOs, adapted from [1].
Q: Are there any significant differences between the real and LCAO MOs?
There are no significant differences between the real and LCAO MOs as can be observed in the MO diagram above.
Q: What does this say about the accuracy and usefulness of qualitative MO theory?
This says that qualitative MO theory is actually rather accurate and useful in predicting the real life molecular orbitals.
jmol of BH3
BH3 molecule |
NH3
Optimisation using 6_31G (d,p) , B3LYP
A 6_31G optimisation with B3LYP method was also run for the NH3 molecule. The run has successful completed as can be seen from the low gradient below 0.001 a.u. and convergence as seen from the Item table below.
-
NH3 results summary for optimisation with 6-31G(d,p) basis set
Link to log file: File:CN_NH3_631G_OPT.LOG
Extract of Item table from .log file ː
Item Value Threshold Converged?
Maximum Force 0.000005 0.000450 YES
RMS Force 0.000003 0.000300 YES
Maximum Displacement 0.000010 0.001800 YES
RMS Displacement 0.000007 0.001200 YES
Predicted change in Energy=-7.830786D-11
Optimization completed.
-- Stationary point found.
Frequency analysis
As seen from the Summary below, the total energy is the same at -56.55777 a.u. to the optimisation run.
-
NH3 results summary for the frequency run
Link to the log file: File:CN_NH3_FREQ.LOG
Extract of the "low frequencies" from the .log file
Low frequencies --- -11.6527 -11.6490 -0.0048 0.0332 0.1312 25.5724 Low frequencies --- 1089.6616 1694.1736 1694.1736
The lowest frequency range is within the ±50 cm-1 range, the formally zero frequencies are well separated from the lowest energy positive frequency at 1089 cm-1 and the large formally zero frequencies are due to the low level of the basis set and relatively relaxed convergence and integration criteria, which can occur for small molecules like NH3.
Item Value Threshold Converged?
Maximum Force 0.000005 0.000450 YES
RMS Force 0.000003 0.000300 YES
Maximum Displacement 0.000012 0.001800 YES
RMS Displacement 0.000006 0.001200 YES
Predicted change in Energy=-8.435065D-11
Optimization completed.
-- Stationary point found.
Vibrational spectrum for NH3
| Wavenumber(cm-1) | Intensity (Arbitrary units) | Symmetry | IR active? | type |
|---|---|---|---|---|
| 1089 | 538 | A | Yes | Symmetric bend |
| 1694 | 50 | E | Yes | Asymmetric bend |
| 1694 | 50 | E | Yes | Asymmetric bend |
| 3461 | 4 | A | No | Symmetric stretch |
| 3589 | 1 | E | No | Asymmetric stretch |
| 3589 | 1 | E | No | Asymmetric stretch |
Snapshot of the spectrum:
-
NH3 IR spectrum
There are less than 6 peaks in the spectrum. This is because the 2nd, 3rd vibrations are degenerate, and so ar the 4th and 5th vibrations in the table, for within these pairs, the vibrations have the same wavenumber and intensity given that wavenumber is proportional to energy. Because they are degenerate and of the same energy, they give rise to a single peak in the spectra. The last two three vibrations (of which two are degenerate) have very low relative intensities and these vibrations will not be experimentally observable. Hence only two peaks corresponding to the first three vibrations in the table (of which two are degenerate), will be observed.
jmol of NH3
NH3 molecule |
NH3-BH3
Optimisation using 6_31G, B3LYP
A 6_31G optimisation with B3LYP method was also run for the NH3 molecule. The run has successfully completed as can be seen from the low gradient below 0.0001 and convergence as seen from the Item table below.
-
NH3-BH3 results summary
Link to the log file: File:CN_NH3BH3_OPT.LOG
Item Value Threshold Converged?
Maximum Force 0.000139 0.000450 YES
RMS Force 0.000063 0.000300 YES
Maximum Displacement 0.000771 0.001800 YES
RMS Displacement 0.000338 0.001200 YES
Predicted change in Energy=-2.028054D-07
Optimization completed.
-- Stationary point found.
Frequency analysis
As seen from the Summary below, the total energy is the same at -83.22469 a.u. to the optimisation run.
-
NH3-BH3 results summary for the frequency run
Link to the log file: File:CN_NH3BH3_FREQ.LOG
Extract of the "low frequencies" from the .log file
Low frequencies --- -0.0613 -0.0448 -0.0067 22.1060 22.1116 40.5984 Low frequencies --- 265.9056 632.3740 640.1221
The lowest frequency range is within the ±50 cm-1 range, there are now negative frequencies and hence this frequency analysis is accepted.
Item Value Threshold Converged?
Maximum Force 0.000121 0.000450 YES
RMS Force 0.000067 0.000300 YES
Maximum Displacement 0.000779 0.001800 YES
RMS Displacement 0.000426 0.001200 YES
Predicted change in Energy=-2.165787D-07
Optimization completed.
-- Stationary point found.
Vibrational spectrum for NH3-BH3
| Wavenumber(cm-1) | Intensity (Arbitrary units) | Symmetry | IR active? | type |
|---|---|---|---|---|
| 265 | 0 | A2 | No | |
| 632 | 5.6 | A1 | Slightly | |
| 640 | 1.4 | E | Very slightly | |
| 640 | 1.4 | E | Very slightly | |
| 1069 | 16.2 | E | Yes | |
| 1069 | 16.2 | E | Yes | |
| 1196 | 43.6 | A1 | Yes | |
| 1203 | 1.4 | E | Very slightly | |
| 1203 | 1.4 | E | Very slightly | |
| 1330 | 45.4 | A1 | Yes | |
| 1676 | 11 | E | Slightly | |
| 1676 | 11 | E | Slightly | |
| 2470 | 26.9 | A1 | Yes | |
| 2530 | 92.5 | E | Yes | |
| 2530 | 92.5 | E | Yes | |
| 3462 | 1 | A1 | very very slightly | |
| 3579 | 11.2 | E | Slightly | |
| 3579 | 11.2 | E | Slightly |
Q: Look at your number, is it a sensible value? How do you know what a sensible value is? (Hint: this is a bond energy, so what "ballpark" value should it have?
This is a sensible value, as it comes close to the single bond dessociation energies that are usually within the range of 150–400 kJ/mol[2]
Also it is a negative value as it should be, as no bonds are being broken but a bond between B and N is being formed, which will then be exothermic.
Q: Based on your energy calculation is the B-N dative bond weak, medium or strong? What comparison have you made to come to this conclusion?
Based on the energy calculations, the B-N dative bond is weak, as it is lower in magnitude than the B-N bond dissociation energy[3] at 377.9 kJmol-1, which is taken from a molecule in which there is a B-N single covalent bond.
Smf115 (talk) 16:21, 26 May 2018 (BST)Good comparison made but there doesn't seem to be any calculation of the association energy itself.
jmol of NH3-BH3
NH3-BH3 molecule |
BBr3
Optimisation using pseudo potentials
-
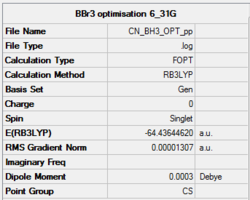
Summary of results for optimisation of BBr3</suɓ> using pseudo-potentials.
Link to log fileː File:CN_Bbr3_OPT_PP.LOG
Item table shows convergence and a successful run.
Item Value Threshold Converged?
Maximum Force 0.000030 0.000450 YES
RMS Force 0.000012 0.000300 YES
Maximum Displacement 0.000154 0.001800 YES
RMS Displacement 0.000080 0.001200 YES
Predicted change in Energy=-3.282217D-09
Optimization completed.
-- Stationary point found.
Frequency Analysis
As seen from the Summary below, the total energy is the same at -64.43645 a.u. to the optimisation run.
-
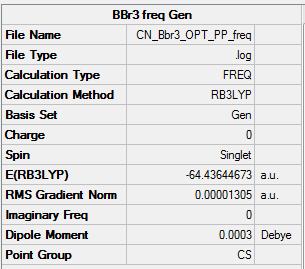
BBr3 results summary for the frequency run
Link to the log file: File:CN_BBR3_OPT_PP_FREQ.LOG
Extract of the "low frequencies" from the .log file
Low frequencies --- -5.6583 -3.3109 -2.2850 -0.0002 0.0001 0.0002 Low frequencies --- 155.8429 155.9360 267.6978
The lowest frequency range is within the ±50 cm-1 range, and hence this frequency analysis is accepted.
Item Value Threshold Converged?
Maximum Force 0.000028 0.000450 YES
RMS Force 0.000013 0.000300 YES
Maximum Displacement 0.000106 0.001800 YES
RMS Displacement 0.000044 0.001200 YES
Predicted change in Energy=-2.950687D-09
Optimization completed.
-- Stationary point found.
jmol for BBr3
BBr3 molecule |
Project section: Lewis acids and basesː Al2Cl4Br2
Different isomers and their symmetries
-
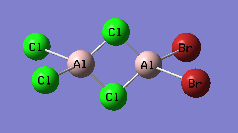
Cl bridging, cis, Cs point group
-
Cl bridging, trans, C2H point group
-
CL bridging, both Br on same Al atom, C2V point group
-
CL,Br bridge, C1 point group
-
Br bridging, D2H point group
Energies
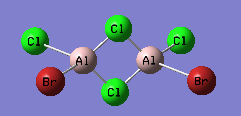
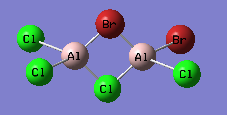
Isomer with trans terminal Br and bridging Cl
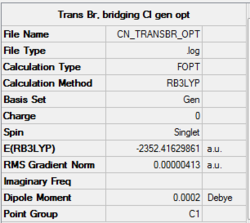
An optimisation using GEN pseudopotentials, B3LYP method was carried out on the built structure. As can be seen from the low gradient of 0.00000413 (<0.001 a.u.) of the run from the summary table below and the convergence as can be seen from the Item table extracted from the .log file, optimisation was complete.
Smf115 (talk) 12:56, 27 May 2018 (BST)Nice detail however, be aware that GEN is not the pseudopotential or the method. It is just an input option to gaussian to tell it that you're using a user-specified basis. The basis set you used was 6-31G(d,p) for Al and Cl and the LanL2DZ basis set and pseudopotential for Br.
-
Summary of results for optimisation of trans isomer using pseudo-potentials.
Item Value Threshold Converged?
Maximum Force 0.000005 0.000450 YES
RMS Force 0.000003 0.000300 YES
Maximum Displacement 0.000053 0.001800 YES
RMS Displacement 0.000023 0.001200 YES
Predicted change in Energy=-6.046439D-10
Optimization completed.
-- Stationary point found.
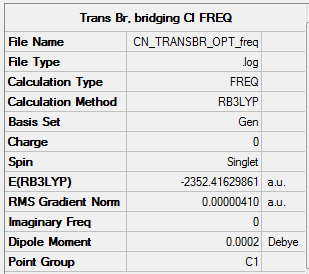
Link to log fileː File:CN_TRANSBR_OPT.LOG
Energy of the isomer was calculated to be -2352.41630 a.u.
A frequency calculation was run using GEN, B3LYP method.
As seen from the Summary below, the total energy is the same at -2352.41630 a.u. to the optimisation run, and the gradient is low at 0.00000410 a.u. (<0.001 a.u.)
-
Results summary for the frequency run
Link to the log file: File:CN_TRANSBR_OPT_FREQ.LOG
Extract of the "low frequencies" from the .log file
Low frequencies --- -5.1504 -0.0036 -0.0021 -0.0011 1.4134 2.0504 Low frequencies --- 18.1470 49.1065 73.0086
The lowest frequency range is within the ±50 cm-1 range, and hence this frequency analysis is accepted.
Item Value Threshold Converged?
Maximum Force 0.000011 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000439 0.001800 YES
RMS Displacement 0.000151 0.001200 YES
Predicted change in Energy=-3.294505D-09
Optimization completed.
-- Stationary point found.
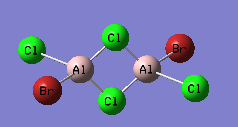
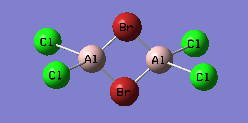
Isomer with two bridging Br
An optimisation using GEN pseudopotentials, B3LYP method was carried out on the built structure. As can be seen from the low gradient of 0.00000182 (<0.001 a.u.) of the run from the summary table below and the convergence as can be seen from the Item table extracted from the .log file, optimisation was complete.
-
Summary of results for optimisation of isomer with bridging Br using pseudo-potentials.
Item Value Threshold Converged?
Maximum Force 0.000003 0.000450 YES
RMS Force 0.000001 0.000300 YES
Maximum Displacement 0.000038 0.001800 YES
RMS Displacement 0.000014 0.001200 YES
Predicted change in Energy=-2.660957D-10
Optimization completed.
-- Stationary point found.
Link to log fileː File:CN_BRIDGEBR_OPT.LOG
Energy of the isomer was calculated to be -2352.40631 a.u.
A frequency calculation was run using GEN, B3LYP method.
-
Summary of results for frequency analysis of isomer with bridging Br using pseudo-potentials.
Low frequencies --- -5.1749 -5.0366 -3.1484 -0.0034 -0.0016 -0.0015 Low frequencies --- 14.8259 63.2702 86.0770
Item Value Threshold Converged?
Maximum Force 0.000006 0.000450 YES
RMS Force 0.000002 0.000300 YES
Maximum Displacement 0.000279 0.001800 YES
RMS Displacement 0.000136 0.001200 YES
Predicted change in Energy=-4.311968D-10
Optimization completed.
-- Stationary point found.
Link to log fileː File:CN_BRIDGEBR_OPT_FREQ.LOG
Relative energies of the isomers
Energy (isomer with trans Br, bridging Cl) -2352.41630 a.u. = -6176269.4661 kJmol-1
Energy(isomer with bridging Br)= -2352.40631 a.u. = -6176243.2374 kJmol-1
|Energy difference|ː 26.22871 kJmol-1
The lower energy isomer is that of the one with bridging CL- ions. This might be because Cl is more electronegative than Br, and hence is better able to stabilise the negative charge as a bridging ligand, than the Br. Additionally, Br is much larger in size (185 pm) compared to Cl (175 pm) given that it has an extra shell of electrons.Hence it would prefer to be at the terminal positions where there is less steric hindrance, having a Br-Al-Br angle of 120 degrees rather than constrained between the two Al atoms where the bond angle is approximately 60 degrees (as in a triangle formation).
The energy difference between the two conformers is very subtle as Br is large and has diffuse orbitals that can still interact with the Al orbitals, and coordinate to them.
Smf115 (talk) 12:54, 27 May 2018 (BST)Correct calculation with a nice justification of the result given.
Dissociation energy of lowest energy conformer
To determine the lowest energy conformer, an optimisation was carried out on the AlCl2Br momomer using the same basis set (GEN, B3LYP).
An optimisation using GEN pseudopotentials, B3LYP method was carried out on the built structure. As can be seen from the low gradient of 0.00004196 a.u. (<0.001 a.u.) of the run from the summary table below and the convergence as can be seen from the Item table extracted from the .log file, optimisation was complete.
-
Summary of results for optimisation of monomer using pseudo-potentials.
Item Value Threshold Converged?
Maximum Force 0.000205 0.000450 YES
RMS Force 0.000096 0.000300 YES
Maximum Displacement 0.001133 0.001800 YES
RMS Displacement 0.000778 0.001200 YES
Predicted change in Energy=-1.597568D-07
Optimization completed.
-- Stationary point found.
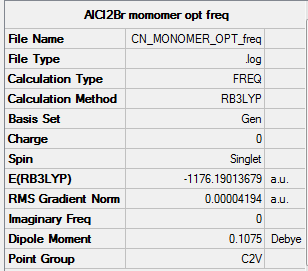
Link to log fileː File:CN_MONOMER_OPT.LOG
Energy of the monomer was calculated to be -1176.19014 a.u. = -3088087.44781 kJmol-1
A frequency calculation was run using GEN, B3LYP method.
As seen from the Summary below, the total energy is the same at -1176.19014 a.u. to the optimisation run, and the gradient is low at 0.00000419 a.u. (<0.001 a.u.)
-
Results summary for the frequency run
Link to the log file: File:CN_MONOMER_OPT_FREQ.LOG
Extract of the "low frequencies" from the .log file
Low frequencies --- 0.0023 0.0033 0.0045 1.3569 3.6367 4.2604 Low frequencies --- 120.5042 133.9178 185.8950
The lowest frequency range is within the ±50 cm-1 range, and hence this frequency analysis is accepted. There are no negative frequencies
Item Value Threshold Converged?
Maximum Force 0.000081 0.000450 YES
RMS Force 0.000042 0.000300 YES
Maximum Displacement 0.001588 0.001800 YES
RMS Displacement 0.000974 0.001200 YES
Predicted change in Energy=-1.810813D-07
Optimization completed.
-- Stationary point found.
Dissociation energy = [ 2(-3088087.44781) -(-6176269.4661) ] kJmol-1 = 94.57048 kJmol-1
The product is more stable than the isolated monomers, given that the energy of the isomer is lower than the sum of two monomers by 94.57048 kJmol-1. This is for the bridging bonds relieve the electron deficiency on the Al atoms and lower the energy of the entire system. Energy must be supplied into the system to break these stabilising bridging bonds and produce the higher energy less stable monomers.
jmols of optimised isomers
jmol of isomer with trans Br, bridging Cl
Isomer with trans Br, bridging Cl |
jmol of isomer with bridging Br
Isomer with bridging Br |
Molecular orbitals of lowest energy conformer (trans Br, bridging Cl)
Three occupied MOs were chosen and analysed as below.
-
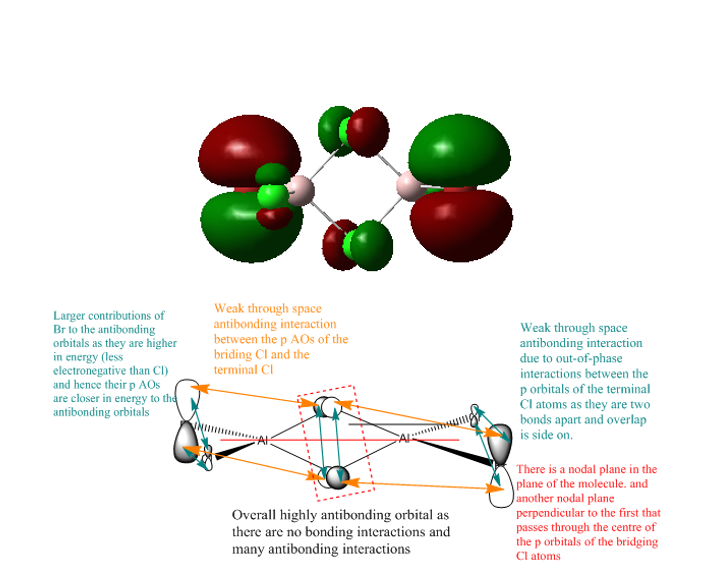
Highly antibonding orbital
-
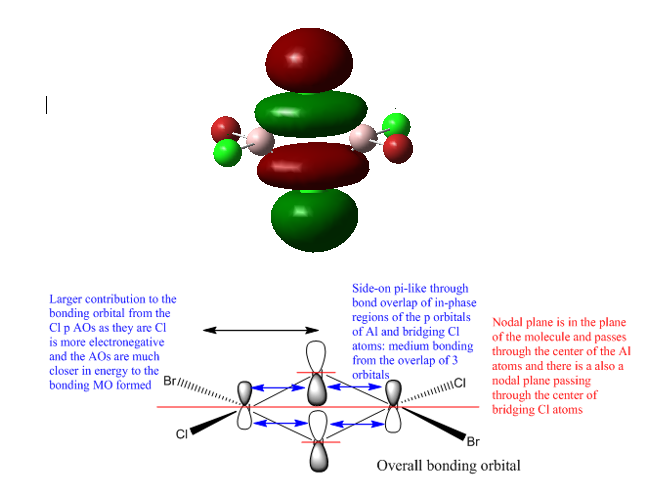
Highly bonding orbital
-
Bonding orbital
Smf115 (talk) 12:53, 27 May 2018 (BST)Good LCAO diagrams and MO analysis with the key interactions labelled and details such as the larger Br contribution is the first MO, and explaination why, included. To improve, the number of the MO visualised should be included and some of the points are incorrect or missed, such as the anti-bonding interaction between the end on p orbtials on the bridging Cl's in the final MO.
Smf115 (talk) 12:53, 27 May 2018 (BST)Overall a good wiki report with nice awareness of the optimisation and frequency procedures and the associated convergence criteria shown.