Rep:Mod:by1517inorganic
EX3 Section
BH3
Computational level and basis set :B3LYP / 6-31G(d,p)

Optimization
Item Value Threshold Converged?
Maximum Force 0.000011 0.000450 YES
RMS Force 0.000007 0.000300 YES
Maximum Displacement 0.000043 0.001800 YES
RMS Displacement 0.000028 0.001200 YES
Predicted change in Energy=-6.856714D-10
Optimization completed.
-- Stationary point found.
Low frequencies
Low frequencies --- -7.5936 -1.5614 -0.0054 0.6514 6.9319 7.1055 Low frequencies --- 1162.9677 1213.1634 1213.1661
Optimized BH3 |
IR
| Wavenumber (cm-1) | IR intensity | Symmetry | IR active | Vibration type |
|---|---|---|---|---|
| 1163 | 93 | A2'' | Y | Out-of-plane wagging |
| 1213 | 14 | E' | Y, not visible | In-plane scissoring |
| 1213 | 14 | E' | Y, not visible | In-plane rocking |
| 2582 | 0 | A1' | N | Symmetrical stretching |
| 2716 | 126 | E' | Y | Asymmetrical stretching |
| 2716 | 126 | E' | Y | Asymmetrical stretching |
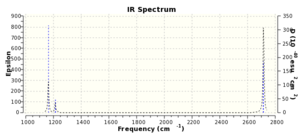
Out of the 6 vibrational modes, there is only 1 inactive mode at 2582 cm-1 as there is no change in dipole moment for this mode. However, the spectrum only show 3 visible peaks. This is due to the 2 degenerate vibration pairs, which are at 1213 cm-1and 2716 cm-1 , these show up as a single peak, adding in a peak at 1163 cm-1, a total of 3 peaks can be seen.
Molecular Orbitals of BH3

NH3
Computational level and basis set :B3LYP / 6-31G(d,p)
Optimization
Item Value Threshold Converged?
Maximum Force 0.000006 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000012 0.001800 YES
RMS Displacement 0.000008 0.001200 YES
Predicted change in Energy=-9.843521D-11
Optimization completed.
-- Stationary point found.
Optimization + Frequency analysis log file
Low frequencies
Low frequencies --- -0.0129 -0.0024 -0.0016 7.1031 8.1045 8.1048
Low frequencies --- 1089.3834 1693.9368 1693.9368
Optimized NH3 |
NH3BH3
Computational level and basis set :B3LYP / 6-31G(d,p)

Optimization
Item Value Threshold Converged?
Maximum Force 0.000121 0.000450 YES
RMS Force 0.000057 0.000300 YES
Maximum Displacement 0.000564 0.001800 YES
RMS Displacement 0.000316 0.001200 YES
Predicted change in Energy=-1.705954D-07
Optimization completed.
-- Stationary point found.
Low Frequencies
Low frequencies --- -0.0253 -0.0034 -0.0012 17.0275 17.0301 36.9251
Low frequencies --- 265.7528 632.2151 639.3365
Optimized NH3BH3 |
E(NH3 ) = - 56.56 a.u.
E(BH3 ) = - 26.63 a.u.
E(NH3BH3) = - 83.22 a.u.
ΔE = E(NH3BH3)-[E(NH3)+E(BH3)] = -0.052 a.u. = -136.526 kJ mol-1
Compared to the C-C bond which has a bond strength of 347 kJ mol-1[1], the B-N bond is very weak.
NI3
Computational level and basis set :B3LYP / 6-31G(d,p)

Optimization
Item Value Threshold Converged?
Maximum Force 0.000068 0.000450 YES
RMS Force 0.000044 0.000300 YES
Maximum Displacement 0.000493 0.001800 YES
RMS Displacement 0.000333 0.001200 YES
Predicted change in Energy=-5.826109D-08
Optimization completed.
-- Stationary point found.
Optimization + Frequency analysis log file
Low frequencies
Low frequencies --- -12.7380 -12.7319 -6.2907 -0.0039 0.0188 0.0633
Low frequencies --- 101.0326 101.0333 147.4124
Optimized NI3 |
The optimized N - I distance is 2.18362 Å
Project section - Ionic Liquids
[N(CH3)4]+
Computational level and basis set :B3LYP / 6-31G(d,p) LANL2DZ
C - N bond length : 1.509 Å
C - H bond length : 1.090 Å
Optimization
Item Value Threshold Converged?
Maximum Force 0.000068 0.000450 YES
RMS Force 0.000027 0.000300 YES
Maximum Displacement 0.000155 0.001800 YES
RMS Displacement 0.000067 0.001200 YES
Predicted change in Energy=-9.049902D-08
Optimization completed.
-- Stationary point found.
Optimization + Frequency analysis log file
Low Frequency
Low frequencies --- 0.0001 0.0008 0.0008 22.7173 22.7173 22.7173
Low frequencies --- 190.7547 294.0687 294.0687
Optimized BH3 |
[P(CH3)4]+
Computational level and basis set :B3LYP / 6-31G(d,p)
C - P bond length : 1.817 Å
C - H bond length : 1.093 Å
Optimization
Item Value Threshold Converged?
Maximum Force 0.000128 0.000450 YES
RMS Force 0.000032 0.000300 YES
Maximum Displacement 0.000666 0.001800 YES
RMS Displacement 0.000277 0.001200 YES
Predicted change in Energy=-1.587537D-07
Optimization completed.
-- Stationary point found.
Optimization + Frequency analysis log file
Low Frequency
Low frequencies --- 0.0018 0.0022 0.0029 26.3168 26.3168 26.3169
Low frequencies --- 160.9764 195.4756 195.4756
Optimized P(CH3)4+ |
[N(CH3)4]+ vs [P(CH3)4]+ charge distributions
| Charge on Carbon | Charge on Hydrogen | Charge on Central atom | |
|---|---|---|---|
 |
-0.48 | 0.27 | -0.30 |
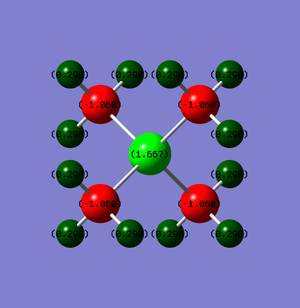 |
-1.06 | 0.30 | 1.67 |
In both molecules, carbon carries negative charges and hydrogen carries positive charges. In [N(CH3)4]+, nitrogen is the most electronegative atom within this ion so a negative charge is expected. The charge analysis does show nitrogen carrying a negative charge. However, the carbon atoms actually carry most of the negative charge and individual carbon atoms are more negative than the nitrogen atom. This is perhaps due to the need for nitrogen to donate its lone pair for a dative covalent bond with a methyl carbocation. This leaves nitrogen with less negative charge. In addition with 3 electropositive hydrogens attached to each of the carbon, carbons would be the most negatively charged atom in the ion. This leaves each of the hydrogens with about a quarter of a positive charge
For [P(CH3)4]+, phosphorus is the most electropositive atom in the ion, so the carbon atoms would pull electron density from phosphorus in addition to the donation of the phosphorus' lone pair to a methyl group, leaves the phosphorus atom very positively charged at 1.67. Conversely, carbon being the most electronegative, carries the negative charge in the molecule, each holding -1.06 due to the pulling of electron density from phosphorus and hydrogen. The hydrogens, like in [N(CH3)4]+, are all similarly positively charged, with only slightly more positive charge. In comparison with the central atom as N, P is actively pushing electrondensity away so that the carbon atoms are only pulling electron density towards it whereas with N carbon is pulling electron density from H and pushing electron density to N.
In the traditional depiction, the formal positive is assigned to the nitrogen. This charge is assigned assuming that all of the electrons in bonds are shared equally among all atoms, disregarding the difference in electronegativity between atoms. Since nitrogen have donated an entire lone pair to form a σ bond, the positive charge is assigned to it. However, charge analysis shows that this is not the case and in fact the positive charge is distributed among the H atoms. Because of this distribution of charge, there are neutral regions within the ions between the positively charged and negatively charged atoms. In [N(CH3)4]+ , there are neutral regions between C and H atoms while in [P(CH3)4]+ there is an additional region between P and C atoms.
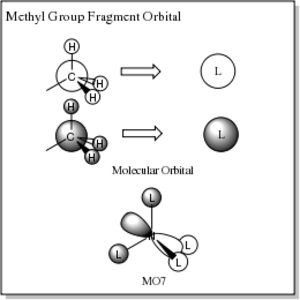
[N(CH3)4]+ Molecular Orbitals
| MO number | Energies (Hartree) | MO visualization | LCAO Drawing | 3D model of MO | |||
|---|---|---|---|---|---|---|---|
| MO7 | -0.93 | 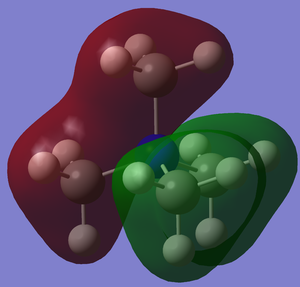
|
 |
| |||
| MO15 | -0.62 | 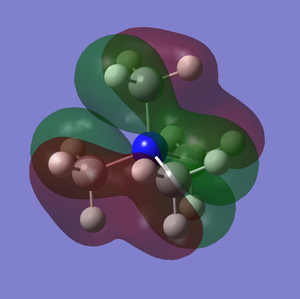 |
 |
| |||
| MO16 | -0.58 |  |
 |
|
