Rep:Mod:bs46182018
Optimised NH3
Item table
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000072 0.001800 YES
RMS Displacement 0.000035 0.001200 YES
Predicted change in Energy=-5.986280D-10
Optimization completed.
-- Stationary point found.
Information about the optimisation
| Molecule | NH3 |
|---|---|
| Calculation method | RB3LYP |
| Basis set | 6-31G(d,p) |
| E(RB3LYP) in au | -56.55776873 |
| RMS gradient | 0.00000485 |
| point group | C3V |
Bond length and angle
Bond length: 1.02 Å. Experimental value: 1.012 Å[1]
Bond angle (H-N-H): 106°. Experimental value: 106.67°[1]
jmol file
ammonia |
Link to the log file here
Vibrations
Screenshot of Display Vibrations
Table of vibrations
| Wavenumber
cm-1 |
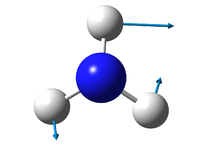
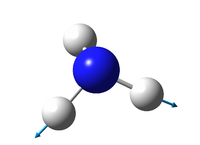
1090 | 1694 | 1694 | 3461 | 3590 | 3590 |
| symmetry | A1 | E | E | A1 | E | E |
| Intensity
arbitrary units |
145 | 14 | 14 | 1.1 | 0.27 | 0.27 |
| image | 
|
|

|

|

|
|
Expected modes from the 3N-6 rule:
3 x 4 - 6 = 6
Degenerate modes:
1694 cm-1 (2)
3590 cm-1 (2)
Bending vibrations:
1090 cm-1
1694 cm-1
Bond stretches:
3461 cm-1
3590 cm-1
Umbrella mode:
1090 cm-1
Experimental spectrum of gaseous ammonia:
4 absortption bands will appear as each vibration induces a change in dipole moment because the ammonia is asymmetric, with permanent dipole moment. 2 degenerate frequencies are present, which results only 4 distinct absorption bands.
Charge analysis
charge on the N-atom: -1.125 Debyes
charge on the H-atom: 0.375 Debyes
The nitrogen is negative as expected because its electronegativity is higher than the hydrogen's (and higher ionisation energy, more negative electronaffinity).
Optimised H2
Item table
Item Value Threshold Converged?
Maximum Force 0.000000 0.000450 YES
RMS Force 0.000000 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000001 0.001200 YES
Predicted change in Energy=-1.164080D-13
Optimization completed.
-- Stationary point found.
Information about the optimisation
| Molecule | H2 |
|---|---|
| Calculation method | RB3LYP |
| Basis set | 6-31G(d,p) |
| E(RB3LYP) in au | -1.17853936 |
| RMS gradient | 0.00000017 |
| point group | D∞h |
Bond length
Bond length: 0.74 Å Experimental value: 0.741 Å[2]
jmol file
hydrogen |
Link to the log file here
Vibrations
Screenshot of Display Vibrations
Table of vibrations
| Wavenumber
cm-1 |
4466 |
| symmetry | SGG |
| Intensity
arbitrary units |
0 |
| image | 
|
Charge analysis
charge on the H-atom: 0 Debyes
Optimised N2
Item table
Item Value Threshold Converged?
Maximum Force 0.000001 0.000450 YES
RMS Force 0.000001 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000000 0.001200 YES
Predicted change in Energy=-3.401013D-13
Optimization completed.
-- Stationary point found.
Information about the optimisation
| Molecule | N2 |
|---|---|
| Calculation method | RB3LYP |
| Basis set | 6-31G(d,p) |
| E(RB3LYP) in au | -109.52412868 |
| RMS gradient | 0.00000060 |
| point group | D∞h |
Bond length
Bond length: 1.11 Å Experimental value: 1.098 Å[3]
jmol file
nitrogen |
Link to the log file here
Vibrations
Screenshot of Display Vibrations
Table of vibrations
| Wavenumber
cm-1 |
2457 |
| symmetry | SGG |
| Intensity
arbitrary units |
0 |
| image | 
|
Charge analysis
charge on the N-atom: 0 Debyes
Trans-bis(Dinitrogen)-bis(1,2-bis(bis(4-ethylphenyl)phosphino)ethane)-molybdenum
Unique identifier: DAMSOA
N-N Bond lengths: 1.116(2) Å, 1.117(2) Å
These experimental bond lengths are longer than in the N2 molecule (Computed to be 1.11 Å). One possible explanation for the difference is that the bond becomes weaker while the molecule forms a complex with the molybdenum atom. The empty dz2 orbital of the metal, and the filled σ - orbital of the nitrogen interact, or a filled d orbital of the metal, and the empty π* - orbital of the nitrogen, forming a new orbital. Both cases weaken the N-N bond, as in the first case electron density is pulled away, in the second case the antibonding character of N-N bond increases, lowering the bond order.[4] Other factor that may contribute to the difference, that the DAMSOA is in a condensed phase, in a crystal structure, where intermolecullar interactions can also affect the bond lengths, unlike in the gaseous N2. The third factor that also can play a role is that experimental, measured values can differ from computed ones because of the errors of both processes.
Ammonia synthesis
This section is about determining the energy for the following reaction:
N2 + 3H2 → 2NH3
| Energy | Value in atomic unit |
|---|---|
| E(NH3) | -56.55777 |
| E(N2) | -109.52413 |
| E(H2) | -1.17854 |
2*E(NH3) = -113.11554 au
3*E(H2) = -3.53562 au
ΔE = 2*E(NH3)-[E(N2)+3*E(H2)]= -0.05579 au = -146.4 kJ/mol The negative change in energy (exothermic reaction) leads to the conclusion that the ammonia is thermodynamically more stable than the gaseous reaction mixture
P4
Molecule of choice Item table
Item Value Threshold Converged?
Maximum Force 0.000000 0.000450 YES
RMS Force 0.000000 0.000300 YES
Maximum Displacement 0.000001 0.001800 YES
RMS Displacement 0.000000 0.001200 YES
Predicted change in Energy=-3.675840D-13
Optimization completed.
-- Stationary point found.
Information about the optimisation
| Molecule | P4 |
|---|---|
| Calculation method | RB3LYP |
| Basis set | 6-31G(d,p) |
| E(RB3LYP) in au | -1365.44182878 |
| RMS gradient | 0.00000016 |
| point group | Td |
Bond lenghth and angle
Bond length: 2.22 Å (each bond) Experimental value = 2.210 Å[5]
Bond angle (P-P-P): 60° (all angles) Experimental value = 60°[5]
jmol file
white phosphorous |
Link to the log file here
Vibrations
Screenshot of Display Vibrations
Table of vibrations
| Wavenumber
cm-1 |
367 | 367 | 464 | 464 | 464 | 607 |
| symmetry | E | E | T2 | T2 | T2 | A1 |
| Intensity
arbitrary units |
0 | 0 | 1.73 | 1.73 | 1.73 | 0 |
| image | 
|

|

|

|

|

|
There are only 3, degenerate vibrations which involve a change in dipole moment in this symmetric non-polar molecule, with a wavenumber of 464 cm -1 and intensity of 1.27. The other 3 symmetric vibration modes do not yield a change in dipole moment therefore they are not IR-active.
Charge analysis
charge on the P-atom: 0 D
Molecular orbitals of P4
In this section 5 MO-s will be presented of this molecule.
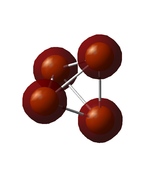
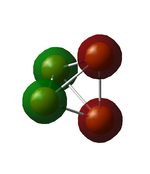
5th MO
Mostly the 2s atomic orbitals contribute to this orbital. The ground state contribution of phosphorous is 1s2 2s2 3s2 3p3, so 2s electrons are not part of the valence shell. This can be seen on the visualisation, as there is no spatial interaction between the orbitals. The energy of the orbital is -6.5809 in au. The information about the atomic orbital contribution was obtained from the .log file (link above).
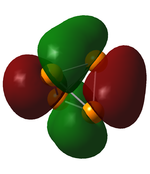
6th-8th MOs
3 Degerenate orbitals with the same energies and shape. The contributing orbitals are the same for these MOs, as of the previous one. 2-2 contributing AOs are in the opposite phase now, but the energy is only a little bit higher, -6.5808 au (0.0001 au difference). This is a proof for the minimal amount of interactions between the 2s orbitals.
21st MO
This bonding orbital builds up mostly from the contribution of the 3s atomic orbitals, but the 2s also contribute some. This is the lowest energy orbital with significant contribution from the valence shell, with an energy of -0.8154 au.
30th MO
This is the highest occupied molecular orbital (HOMO) of the molecule. It has a partial bonding and a partial antibonding character, as there are radial nodes on 2 bonds, but 4 bonds with in-phase orbital overlap. It is formed from the overlap of 3p orbitals, with no contribution from the 3s. The energy of the HOMO -0.2721 au.

31st MO
This is the lowest unoccupied molecular orbital (LUMO) of the molecule. It's an antibonding orbital with radial nodes on all bonds. It is built from the contribution of 4px and 4pz orbitals but with significant contribution of the corresponding 3p and 2p orbitals. The energy of the HOMO -0.0376 au. The energy of the LUMO is negative which means it's thermodynamically favoured to act as an electrophile.
References
- ↑ 1.0 1.1 Herzberg, G., Electronic spectra and electronic structure of polyatomic molecules,Van Nostrand,New York, 1966
- ↑ Huber, K.P.; Herzberg, G., Molecular Spectra and Molecular Structure. IV. Constants of Diatomic Molecules,, Van Nostrand Reinhold Co., 1979
- ↑ Huber, K.P.; Herzberg, G., Molecular Spectra and Molecular Structure. IV. Constants of Diatomic Molecules,, Van Nostrand Reinhold Co., 1979
- ↑ Rob Toreki, 2015 http://www.ilpi.com/organomet/alkene.html
- ↑ 5.0 5.1 LR Maxwell, SB Hendricks, VM Mosley "Electron Diffraction by Gases" J. Chem. Phys. 3, 699, 1935 doi: 10.1063/1.1749580
Marking
Note: All grades and comments are provisional and subject to change until your grades are officially returned via blackboard. Please do not contact anyone about anything to do with the marking of this lab until you have received your grade from blackboard.
Wiki structure and presentation 1/1
Is your wiki page clear and easy to follow, with consistent formatting?
YES
Do you effectively use tables, figures and subheadings to communicate your work?
YES
NH3 0.5/1
Have you completed the calculation and given a link to the file?
YES
Have you included summary and item tables in your wiki?
YES
Have you included a 3d jmol file or an image of the finished structure?
YES
Have you included the bond lengths and angles asked for?
YES
Have you included the “display vibrations” table?
YES
Have you added a table to your wiki listing the wavenumber and intensity of each vibration?
YES
Did you do the optional extra of adding images of the vibrations?
YES
Have you included answers to the questions about vibrations and charges in the lab script?
YES - You correctly gave the reason for 4 modes. You missed to interpret the intensities. The stretching vibrations are too low in intensity to be observed. Only two band are expected.
N2 and H2 0.5/0.5
Have you completed the calculations and included all relevant information? (summary, item table, structural information, jmol image, vibrations and charges)
YES
Crystal structure comparison 0.5/0.5
Have you included a link to a structure from the CCDC that includes a coordinated N2 or H2 molecule?
YES
Have you compared your optimised bond distance to the crystal structure bond distance?
YES
Haber-Bosch reaction energy calculation 1/1
Have you correctly calculated the energies asked for? ΔE=2*E(NH3)-[E(N2)+3*E(H2)]
YES
Have you reported your answers to the correct number of decimal places?
YES
Do your energies have the correct +/- sign?
YES
Have you answered the question, Identify which is more stable the gaseous reactants or the ammonia product?
YES
Your choice of small molecule 3/5
Have you completed the calculation and included all relevant information?
YES
Have you added information about MOs and charges on atoms?
YES
You correctly identified contributing AOs. You missed to to explicitly state the effect on bonding and the occupancy. You could have discussed the energies of the MOs in more detail.
You could have explained the calculated charges and mention how many modes were expected.
Independence 1/1
If you have finished everything else and have spare time in the lab you could:
Check one of your results against the literature, or
YES - But your comparisons are minimal.
Do an extra calculation on another small molecule, or
Do some deeper analysis on your results so far