Rep:Mod:brapbrap
Module 1 - Organic: Exercise 1
The main purpose of exercise 1 was to become familiar with two of the basic tools of molecular modelling, namely ChemBio3D and Gaussview/Gaussian. Molecular Modelling uses molecular mechanics in order to predict the energy and orientations of 3D molecules. Molecular Mechanics itself is based on one key assumption which is that the energy of a system can be predicted by considering three aspects of the system, these being the strain, sterics and hydrogen bonding present in the molecule. In total molecular mechanics gives predicted energies made up of the following additive terms:
- Sum of Diatomic Bond Stretches
- Sum of Triatomic bond Angle Deformations
- Sum of Tetraatomic Bond Torsions
- Sum of non-bonded Van der Waals Repulsions
- Sum of Electrostatic attractions of Individual Bond Dipoles
Molecular modelling is, like most models and approximations, not perfect. The main limitations of the processes are the fact that the models only show thermodynamic control and also that the models show no knowledge of the electron distribution within the molecule.
The Hydrogenation of Cyclopentadiene Dimer
This study involves the π4s + π2s cycloaddition of cyclopentadiene to form dimers of the original species, these can obviously be either exo or endo. Additionally the hydrogenation of the dimer will be modelled.
By the use of ChemBio 3D the relative energies of the species will be determined and by looking at these results it is hoped that the an understanding of the mechanistic rationale and the method of control, either kinetic or thermodynamic, can be determined for both the dimerisation and the subsequent hydrogenation.
In order to determine the relative energies the molecules were drawn as 2D ChemDraw files, these were automatically converted to 3D structures by the program. This then allowed an MM2 calculation to be run to determine the lowest energy conformation for each species, the below table shows the 3D molecules in their lowest energy configurations as well as indicating what the lowest energy is.
Table 1:Cyclopentadiene Dimers Relative Energies | |||||||||||||||
| Exo Form | Endo Form | Hydrogenated Isomer 1 | Hydrogenated Isomer 2 | ||||||||||||
|
|
|
| ||||||||||||
| 31.8832kcal/mol | 34.0152 kcal/mol | 35.9334 kcal/mol | 31.1540 kcal/mol | ||||||||||||
Dimerisation
It is generally accepted that the dimerisation of cyclopentadiene results in the formation of the endo product. On first glance this seems counterintuitive as we notice that the exo product is of lower energy by 2.132kcal/mol which is a significant amount. Looking at this quantitatively we would expect the exo product to be more stable due to its proximity to a staggered structure, which is fundamentally more stable due to the absence of the repulsive interactions of the bonding electrons as well as the favourable σ-σ* orbital overlap of app bonds. When we look at the results generated from the minimisation we can immediately see the main difference between the two molecules is the torsional strain, mainly centred at the newly formed C-C bond between the two molecules making up the dimer. This torsional strain is 9.5039 kcal/mol for the endo product, compared with 7.6715 kcal/mol for the exo product and so this can be seen as the main reason behind the significant difference in energy between the two molecules.
Despite the exo product being notably more stable, this is not the product we see in the reaction, leading us to the obvious conclusion that the reaction is in fact kinetically, and not thermodynamically controlled. The upshot of this is that we can say that the reaction proceeds by the lowest energy pathway, through the most stable transition state. As such to further understand the reason for the endo formation we must look into the transition states of each of the two dimers to see why the endo transition state is of lower energy. In looking at the transition states of the two molecules and also viewing literature references [1] the reason behind this lower energy transition state for the endo product is secondary orbital interactions. Secondary orbital interactions involve orbitals which are not involved in bonding but have a favourable overlap in the transition state, thus lowering the overall energy of the transition state, lowering the energy of the pathway. An example of secondary orbital interactions is shown below[2]:
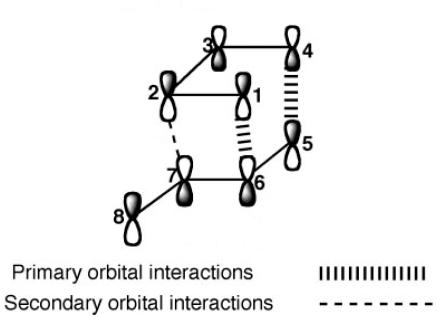
As such it can be seen that due to an increased number of SOI (secondary orbital interactions) in the endo formation, this will be the favourable product, explaining why we see endo formation in the dimerisation studied.
Hydrogenation
Most basically we can look at the relative energies of the two molecules and see a difference of 4.7794kcal/mol, which is not an insignificant difference. Molecule 4 is thermodynamically more stable than molecule 3, and so from first principles this would be expected as the product of hydrogenation. The reason for this higher stability appears to be the decrease in the bend energy when looking at the outputs. For 3 the bending energy is 18.8641kcal/mol, whereas for 4 this is 14.5074kcal/mol.
When looking at the actual molecules the fact that 4 is seen as more stable makes sense as in hydrogenating the double bond closest to the methyl bridge, ie. that in 4, the reduction occurs to the most strained double bond. This is fundamentally the more favourable of the reductions due to it giving the most relief of strain, this is indeed reflected in the energies. Hence molecule 4 predominates over molecule 3 in the hydrogenation of the dimer.
Stereochemistry of Nucleophilic Additions
The idea behind this part of the study was to study two reactions by molecular modelling in order to establish the lowest energy conformation of the reactants. The result of this is then used to rationalise the stereochemistry observed in the product.
Attack of A Grignard Reagent | |||
| |||
| |||
| 1,4 van der Waals = 11.8751kcal/mol | |||
| Bend = 11.3684kcal/mol | |||
| Total Energy = 26.4711 kcal/mol | |||
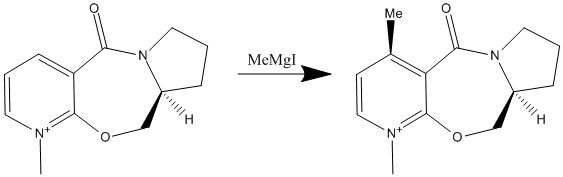
The first reaction modelled was the attack of a Grignard reagent, which is one which has been studied in literature[3]
Molecule 5 was modelled first. This modelling did not include the Grignard reagent as this could not be recognised by the program, this is due to the program being unable to rationalise the structures of metal ions due to the lack of availability of force constants for the bonds. The molecule which was modelled started with the initial stereochemistry given by the 2D structure.
Minimisation of the energy was simple to obtain and when moving the carbonyl groups interesting results were observed. The optimal angle is approximately 40°, leading to the energy reported. When enforcing a small bond angle the molecule increased in energy, additionally when the carbonyl group was moved up and down no matter where the group was moved to the group was always optimised to move above the ring, leading to the observation that this is the optimum conformation, making this the most favourable position for the carbonyl group, as such this is can be considered as the enforced, and so only conformation of the molecule.
The stereochemical implication of this enforced conformation is that due to the position of the carbonyl group there is only one way in which the Nucleophilic Grignard reagent can attack, that being below the plane of the ring resulting in only one stereochemical outcome, the formation of the observed product 6.
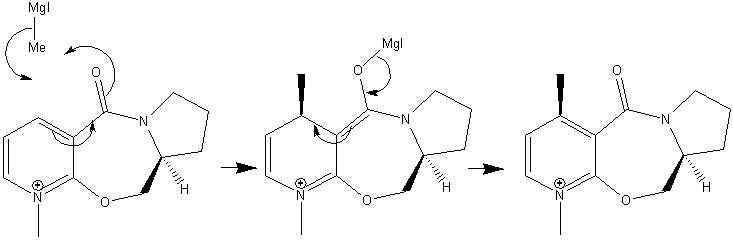
Above is the proposed reaction mechanism, as shown the methyl group adds stereospecifically to one face of the ring, resulting in a stereospecific product. The reason for this stereospecificity appears to be the co-ordination of the metal to the oxygen atom, lowering the energy of the transition. This very favourable interaction means the reaction is shown to be stereospecific generating only one product.
The second reaction studied was a similar reaction with aniline which showed similar features of selectivity. The modelled molecule and significant contributions to the energy are indicated below.
Reaction with Aniline | |||

| |||
| |||
| 1,4 van der Waals = 17.5328kcal/mol | |||
| Bend = 6.6014kcal/mol | |||
| Total Energy = 15.4380kcal/mol | |||
The reactant molecule for this reaction was again drawn and minimised with the minimum energy being when the carbonyl group is below the plane of the ring. Attempts were then made to minimise the energy further. The main approach to this was by the movement of the carbonyl group to above and below the ring in order to find the lowest energy conformation. Upon moving the carbonyl group above the ring, the resulting optimisation was apparent inversion to form a structure analogous to the original structure.
As such we again appear to have an enforced configuration for the minimisation of energy, with the carbonyl group below the ring. In contrast to the first reaction we see no possibility of co-ordination to the metal and so this clearly renders no control over the reaction. The nucleophile in this case is PhNH2. This is sterically large due to the phenyl group and so sterics will render control in the reaction. As previously discussed the carbonyl is found on the bottom face, hence the nucleophile will attack from the top face. This results in the observed stereochemistry of the product.
Possible Improvements
The main limitations of Molecular Modelling with ChemBio3D is the basis of the program. This is that the program is based on already well known and established molecular interactions and so works in an interpolative manner, as opposed to extrapolative techniques. This was noticed for the Grignard reagent, where the metal centre was not recognised by the program.
A much better approach would be to consider each molecule and its force constants along with all other possible interactions, this would also involve taking into account the electron distribution, a step up from the current modelling technique. This would mean that new molecules with unusual bonding could be made amenable to modelling.
Examples of such improved programs are Gaussian, which will be used later, as well as Ghemical or the even more modern Avogadro.
Reactivity and Stereochemistry of Key Taxol Intermediates
The third study involved an intermediate involved in the synthesis of Taxol. There were two possible isomers, which are shown in the below table along with a molecular representation of the initially optimised isomers.
| Carbonyl Down | Carbonyl Up | ||||||
|
| ||||||
| Lowest Energy 45.20kcal/mol | Lowest Energy 49.99kcal/mol |
After the representations shown further optimisation occurred to probe the molecules further and reach the lowest energy configuration, as was the aim of the study. As was employed for the previous study the orientation of the carbonyl group was the functionality focused on in the minimisation.
In moving the carbonyl group up and down with reference to the rest of the molecule, the energy of the lowest configuration is given in the above table. The difference in the energies of the two conformers can be rationalised by again looking at the outputs of the two conformers, the result of this is the realisation that the bending energy is the most important factor, accounting for almost all of the difference in the energy of the molecules.
In looking at the molecules the up isomer suffers from a higher bending contribution due to the divergence of the bond angles from 120°. As the angles are significantly less than 120° this increases the bending energy. This follows from theory as the carbonyl carbon is sp2 hybridised and so a reduction in the bonding angles brings the orbitals closer together, resulting in a repulsion and hence an increase in energy, as observed in the output.
Reduced Reactivity
Alkenes of this type are referred to in some quarters as hyperstable[4]. The reason for this increased stability is an interesting issue as this is not immediately obvious. Indeed when the hydrogenated species are modelled their energy is in fact much higher than the starting alkene, showing the process is indeed thermodynamically unfavourable though the reasons for this are still not completely clear.
Literature[5] studies of such hyperstable systems suggest that by hydrogentating the double bond the total energy is increased. This is characterised by see an increase in the torsional strain and Van der Waal's strain. This can be probed further and be seen to be routed in the small dihedral angles generated due to the new C-H bonds and subsequently by the interaction of bonds with the other groups in the molecule. It should further be denoted that such a large resistance to hydrogenation will also mean that any other reactions at the double bond will also be dramatically reduced in rate and even likelihood.
Room Temperature Peptide Hydrolysis
Peptides are the building blocks of proteins and so have a hugely important role in the body. As such these molecules are well regarded for their high stability and resistance to reaction, which is clearly desirable for such critical molecules. However, in stark contrast, the aim of this study is to model room temperature hydrolysis of such molecules.
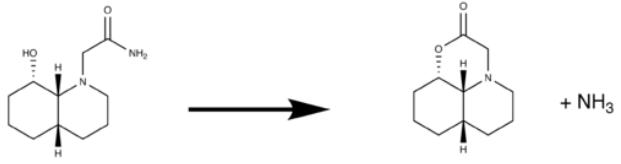
The two molecules involved in this study both have more than one possible isomer and so modelling is important to probe and find the most stable, and so likely, configuration for each molecule involved. It should first be noted that throughout a chair-chair conformation is employs as this is significantly more stable than any molecules containing a boat conformation.
 | |||||||||||||||
| Both Substituents Axial | O Equatorial, N Axial | O Axial, N Equatorial | Both Substituents Equatorial | ||||||||||||
|
|
|
| ||||||||||||
| 29.4232 kcal/mol | 25.6792 kcal/mol | 23.8475 kcal/mol | 20.8268 kcal/mol | ||||||||||||
Looking first at the above reaction involving the cis decalin there are a number of possible configurations depending on whether the substituents are axial or equatorial, the groups which we need to take note of are the N-substituent and the OH functionality. All of the molecules were modelled, again using MM2 methods, and the minimised energy is given in the above table. As can be seen we see the lowest energy for the diequatorial configuration.
This follows from fundamental principles of cyclohexane configurations which suggests the equatorial conformation is more stable than the axial conformation. The reasons for this are well known, these being that the axial substituent suffers a 1,3 diaxial interaction which raises the energy of the conformation making this unfavourable. The difference between the axial and equatorial Gibbs free energy is known as the A value. This defines the preference between axial and equatorial configuration with a positive A value denoting a preference for the equatorial configuration.
This is not the lowest energy configuration as we can probe further, noticing the possibility for hydrogen bonding within the molecule. This is important as such an interaction is stabilising and will therefore reduce the energy of the molecule. The result of this is a lower energy configuration, which has an energy of 16.9493 kcal/mol.
This molecule is given below:
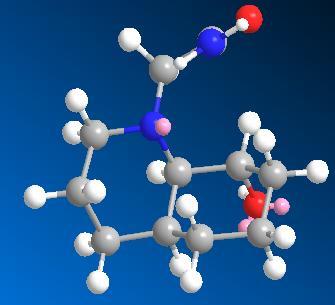
In looking at the final product this will be the conformer from which reaction. When we look at this case with regard to the conformation the molecule does not observe the Burgi-Dunitz angle, suggesting that this is not the most reactive conformation, this can be explained by employing the Curtin Hammett Principle which says that the major conformer in starting material may not be most reactive. This is due to the importance of the Burgi-Dunitz angle which is required for reaction to in fact take place.
This molecule is in fact very reactive and hydrolysis does take place in a kinetic fashion, this has been studied and suggestions for the reasons behind this have included the fact that the molecule is constrained and so as such the reactive conformation has a long lifetime, allowing reaction to take place, so called sustained proximity[6]. Further we can propose that these reactions occur more quickly due to the fact that, due to the intramolecular nature of the reaction, that the rate is increased due to the lack of dependance on the rate of diffusion.
The second reaction involves the trans decalin version of the previous molecule. We can look into the literature [7] for previous studies of this reaction. The reaction scheme is given in the below table along with the lowest energy conformations for the trans decalin system.
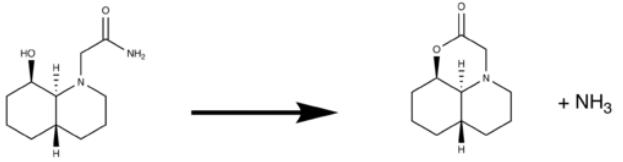 | |||||||
| N Axial | N Equatorial | ||||||
|
| ||||||
| 18.4130kcal/mol | 9.6920kcal/mol | ||||||
As was the case for the cis decalin it is possible to model different conformations with the N-group both axial and equatorial. The equatorial form is again lower in energy, this can again be discussed as being due to the increased stability of the equatorial conformer when compared to the axial conformer. This accounts for the observed difference in energy between the two isomers and suggests that the equatorial conformer will be the dominant conformer in solution.
Again we can look at the reactivity which requires an angle of approximately 109° of attack, this is not attained in the conformers and so again the most stable conformer will not be the most reactive. We can however still see why reaction proceeds quickly, due to the previously discussed intramolecular pathway as well as the “sustained proximity” of the two groups.
One final factor we must consider is why the first reaction is reported to proceed approximately 40 times faster than the second. A simple explanation can be proposed, this is that the molecule is of higher energy, which means that the starting material is of lower energy and so reaching a transition state will require less energy. Additionally we can consider the proximity of the two groups in the first molecule and the fact that they are closer to an angle of 109° and use this to suggest that reaction will be more favourable here. Finally we can consider that in the second reaction the OH group is enforced into the axial position, which means that attaining the desired conformation in a transition state will not be possible.
To summarise, due to the intramolecular pathway and the sustained proximity of the groups reacting in these peptides room temperature hydrolysis is possible in relatively short reaction times.
Modelling Using Molecular Orbital Theory
Part 1
It was now finally time to begin considering the electronic distributions in molecules to take into account the electrons in molecules. This can help to explain such effects as the endo selectivity which was encountered in the first study. Such modelling is done using more advanced programs such as Gaussview.
The process followed was first to optimise using molecular modelling. This resulted in the output given in the below table.
| Stretch | 0.6147 | ||
| Bend | 4.8473 | ||
| Stretch-Bend | 0.0404 | ||
| Torsion | 7.5966 | ||
| Non-1,4 VDW | -1.0849 | ||
| 1,4 VDW | 5.7871 | ||
| Dipole/Dipole | 0.1113 | ||
| Total Energy | 17.9125 kcal/mol | ||
The optimised structure was then used to generate the molecular orbitals using a Hartree Fock approximation. The result of this is the MO images in the below table.
| Di-alkene HOMO | Di-alkene LUMO | ||
|
| ||
| Mono-alkene HOMO | Mono-alkene LUMO | ||
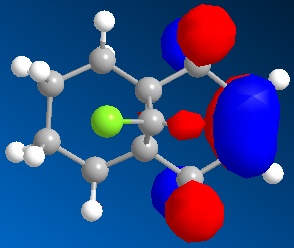
|
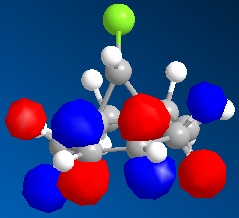
| ||
Part 2
Following this basic modelling process to generate the Molecular Orbitals a more sophisticated approach was followed with the aim of then comparing the resulting orbitals to see the effect of the more advanced technique.
The basic process was to first create the two molecules in Gaussian and then to optimise them using the B3LYP/6-31G(d) basis set, a more advanced approximation than the Hartree Fock approach employed previously.
| Di-alkene HOMO | Di-alkene LUMO | ||
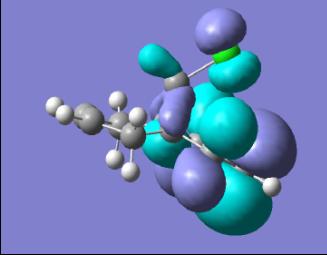
|
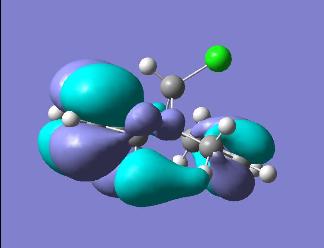
| ||
| Mono-alkene HOMO | Mono-alkene LUMO | ||
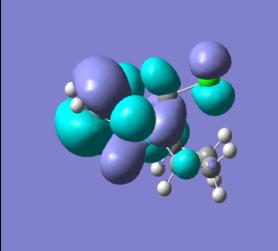
|
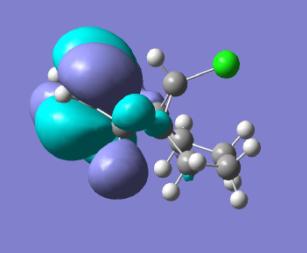
| ||
We can compare these orbitals generated with those described in literature[8] and in doing so find ourselves satisfied that these orbitals correspond to those previously reported.
It is plain to see that the MOs generated by the second, more advanced approach are more advanced and so appear to offer more information with regard to the electronic distribution around the molecule, this is what was predicted before the modelling was carried out and has as such been found to be correct.
In looking at the molecular orbitals for the diene we can gain an understanding of the system and the reactivity it will display. Primarily we can look at the HOMO, this will be the orbital which will be attacked by the dichlorocarbene electrophile. This permits an understanding of where the molecule will be attacked by the electrophile. We can see that in the diene the HOMO is centred on the C-Cl bond and so this is the area of the molecule where the electron density in the electrophilic attack will be found.
In looking at the HOMO the endo C=C is the double bond which is present in the HOMO and so this can be seen as the double bond which will be attacked electrophilically. This makes sense as by having a higher electron density the double bond will be, by definition, more Nucleophilic and so will react much faster.
An orbital explanation from this is the interaction of the pi orbitals in the exo double bond with the antibonding orbitals of the C-Cl bond. This interaction not only stabilises the exo double bond but also removes electron density from it, making it less Nucleophilic and so much less likely to undergo reaction with an electrophile. Further to this a possibly less important interaction is the steric bulk of the chlorine atom which is syn to the endo double bond, this steric repulsion could also make this double bond more reactive in comparison to the exo double bond which has only a hydrogen syn to it.
As such it can be suggested from this simple approach that the product of endo attack will be the dominant, if not sole, product of the reaction with an electrophile, such as dichlorocarbene.
With the monoalkene we see only one double bond, this double bond dominates the HOMO and so this can be seen as the site of any electrophilic attack.
Part3
Both the diene and the monoalkene, which had been previously optimised were then submitted to calculation of the stretching frequencies and vibrations which they could possibly undergo, culminating in the generation of an Infrared Spectrum of the two molecules.
| Key IR Stretches | |||
| C=C Monoalkene | 1740.55 | ||
| C-Cl Monoalkene | 775.577 | ||
| C=C Diene | 1760.95 (endo), 1740.66 (exo) | ||
| C-Cl Diene | 770.091 | ||
The above table shows the key vibrations observed in the IR spectrum, these give us information about the structure and bonding of the molecule as well as interactions within the molecule.
From the fundamental principles of IR Spectroscopy we can infer the strengths of the bonds in the molecule. This is possible due to the fact that the stretching frequency of an absorption is directly proportional to the energy required to stretch or bend the bond, as such the relative strength of the bond can be determined.
Looking first at the C-Cl bonds we can see the interactions occuring. In the above discussion it was hypothesised that there was a donation of pi electon density from the exo double bond into the antibonding orbital of the C-Cl bond. This will have a direct effect on the strength of the C-Cl bond and due to the reduction in bonding character of the bond this will be a weaker bond in this system than an ordinary C-Cl bond. The IR absorptions can tell us a lot about this, as in the diene, where this interaction does take place the C-Cl bond is given as 770.091cm-1, which is in fact lower than the 775.577cm-1 given for the monoalkene. As such the IR Spectrum provides support to the earlier theory that such an interaction between the pi bond and the antibonding C-Cl bond does occur.
Further to this we can look at the two C=C bonds in the diene. As previous we are suggesting a donation of pi electon density from this double bond. This will have the effect of reducing the electron density in this double bond, effectively making it a weaker bond, which will be reflected by a lower wavenumber for the stretch as less energy will be required to stretch the bond. Again we are pleased to see this reflected in the predicted spectrum which gives a significantly lower wavenumber for the exo double bond compared to the endo, again supporting the previous discussion.
In summary the vibrations of the molecule provide support for the earlier proposed interaction involving the exo double bond.
| IR Spectrum of Diene | |||
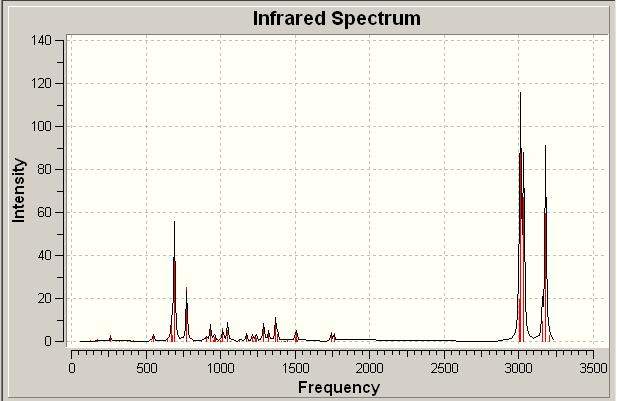
| |||
| IR Spectrum of Alkene | |||
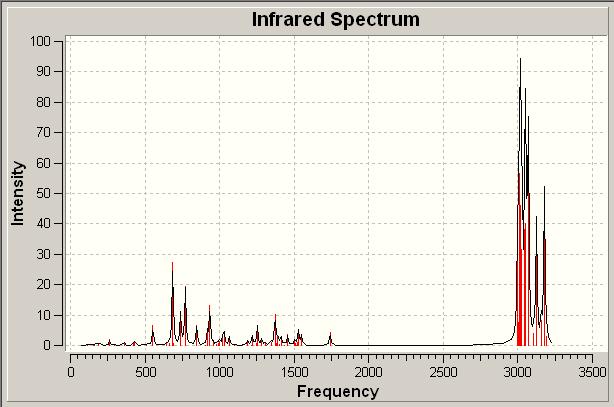
| |||
From the above IR spectra the relative stretches and bends of the molecules can be seen graphically. In both cases the spectra are similar, with the dominant stretches due to the various and numerous possible stretches of the CH bonds which are present in the molecule. In addition to this we also see the previously discussed stretches and bends of the C=C and C-Cl bonds.
In summary, this final study has demonstrated the key uses of more advanced modelling techniques and shown how such methods can yield a plethora of information with regard to the electronic distributions and strengths of the bonds.
This gives us confidence to move further into more advanced techniques with the mini project, available on the following link [2]
References
- ↑ Governing organic reactions through secondary orbital interactions. Semiempirical and density functional theory study of catalyzed cycloaddition reactions between pyrrole and ether dienophiles, B. S. Jursic, J. Chem. Soc., Perkin Trans. 2, 1999, 373–378 [1]
- ↑ Wannere et al.Journal of Computational Chemistry, 2006, Vol. 28, No. 1 DOI:10.1002/jcc.20532
- ↑ A. G. Shultz, L. Flood and J. P. Springer, J. Org. Chemistry, 1986, 51, 838. DOI:10.1021/jo00356a016
- ↑ K. B. Wiberg, Strained Hydrocarbons: Structures, Stability, and Reactivity, Reactive Intermediate Chemistry, 2004, 717-740 DOI:10.1002/0471721492.ch15
- ↑ P. Camps et al, Tetrahedron, 1997, Vol. 53, No. 28, pp. DOI:10.1016/S0040-4020(97)00595-4
- ↑ Menger, F. M.; Ladika, M. J. Am. Chem. Soc. 1988, 110, 6794–6796DOI:10.1021/ja00228a031
- ↑ M. Fernandes, F. Fache, M. Rosen, P.-L. Nguyen, and D. E. Hansen, Rapid Cleavage of Unactivated, Unstrained Amide Bonds at Neutral pH, J. Org. Chem., 2008, 73, 6413–6416 DOI:10.1021/jo800706y
- ↑ B. Halton, R. Boese and H. S. Rzepa., J. Chem. Soc., Perkin Trans 2, 1992, 447. DOI:10.1039/P29920000447
