Rep:Mod:boredofcomplabs
Module 3: Christopher Wood
Apologies for the size of the pictures, time is an issue.
Introduction
In this module, GausView was used not only to optimise and analyse various molecules as before, but now expanded to study transition states. Transition states are states of maximum energy along a reaction path from reactants to products. The structure and geometry of the transition states can significantly determine the structure of the product of the reaction. This is a highly useful tool for chemists and therefore will be investigated in this module
The Cope Rearrangement
In this section, the Cope rearrangement of 1,5-hexadiene will be studied. First of all, the low energy minima were determined for the structural arrangements of 1,5-hexadiene.
The structures were made in GaussView and "Cleaned". The job type was Optimization' and the default method was left unchanged, Hartree Fock with basis set 3-21G. The size of the output files were also limited to a maximum of 250 MB. The resultant molecule's symmetry was noted after selecting Symmetrize. The table below shows the various minima in the structure of 1,5-hexadiene.
| Conformer | Structure | Point Group | Energy/Hartrees HF/3-21G |
Relative Energy/kcal/mol | |||
| gauche1 |
|
C2 | -231.68772 | 3.10 | |||
| gauche2 |
|
C2 | -231.69167 | 0.62 | |||
| gauche3 |
|
C1 | -231.69266 | 0.00 | |||
| gauche4 |
|
C2 | -231.69153 | 0.71 | |||
| gauche5 |
|
C1 | -231.68962 | 1.91 | |||
| gauche6 |
|
C1 | -231.68916 | 2.20 | |||
| anti1 |
|
C2 | -231.69260 | 0.04 | |||
| anti2 |
|
Ci | -231.69254 | 0.08 | |||
| anti3 |
|
C2h | -231.68907 | 2.25 | |||
| anti4 |
ANTI 4 DOI:10042/to-7233 |
C1 | -231.69097 | 1.06 |
The relative energies are taken in comparison to the lowest energy minima, which was determined to be gauche 3.
To compare the energy outcomes of different optimisation methods, the Ci minima structure was reoptimised using B3LYP/3-21G and then 6-31G. There was no observable change in structure, but the energies that were computed were -233.33634 Au and -234.55970 Au respectively.
A frequency job was then run on the optimised structure with the following results: DOI:10042/to-7236
| Hatree | |
| Sum of electronic and zero-point Energies | -234.416244 |
| Sum of electronic and thermal Energies | -234.408953 |
| Sum of electronic and thermal Enthalpies | -234.408009 |
| Sum of electronic and thermal Free Energies | -234.447848 |
Optimising the "Chair" and "Boat" Transition Structures"
The Cope rearrange is a [3,3]-sigmatropic shift rearrangement. it is generally accepted that the reaction occurs in a concerted fashion via either a "chair" or "boat" transition structure. In this section, both structure shall be analysed to a B3LYP/6-31G level of theory, which has been shown to give good agreement to experimental data.
Chair
The first method used to determine the transition structure involved the placement of 2 allyl fragments positioned approximately 2.2 Å apart. They were first optimised using HF/3-21G level of theory. Once the transition structure was approximately made, a Opt+Freq calculation was set up and was optimised to a TS (Berry). The force constants were calculated Once and Opt=NoEigen was added to the Additional keyword box. DOI:10042/to-7246
There was an imaginary frequency observed at -817.89 cm-1 and this corresponded to the bonds forming/breaking in this rearrangement reaction. (Jmol unable to show vibration...)
Vibration |
On examining the structure, the C-C separations, at the sites of the new forming C-C bonds, were observed to be 2.01954 and 2.01999 Å.
The next method investigated involved fixing the C-C separtions mentioned above. First of all, the C-C separations (where the new bonds would form) were fixed at 2.2 Å. An optimisation calculation was run then the separations were then optimised in a second calculation by running an Opt+Freq to a TS (Berry). DOI:10042/to-7267
The C-C separations were noted to be 2.01998 Å. This is similar to the previous method. The structures resulting from both methods were both the same.
Boat
For the boat transition structure a different method had to be employed. this involved mapping the reactants and products and letting the program determine the structure. Initially, a chair TS was given, as below, but after tweaking the geometry of both the reagents and products the boat was achieved. DOI:10042/to-7274 and DOI:10042/to-7275
Again there is only 1 imaginary frequency observed and this corresponds to the desired rearrangement reaction.
Intrinsic Reaction Coordinate
Using IRC, this allows us to follow the minimum energy path from a transition structure down to its local minimum on a potential energy surface. This confirmed that my input chair structure was and TS in accordance to the IRC plot. DOI:10042/to-7277
Energy workup
In this section, the TS's were optimised to B3LYP/6-31G level of theory. The resultant geometries were extremely similar to those already determined above. Chair DOI:10042/to-7280 , Boat DOI:10042/to-7281 .
Unfortunately, due to calculation errors, the energies could not be determined for unknown reasons, even after countless repeats.
Exercise
In this exercise, specific Diels Alder reactions shall be investigated using the methods described above.
Cis-butadiene and Ethylene
Both the molecules were first made separately and optimised to a B3LYP/6-31G level. Their HOMO/LUMO's are as shown:
| Molecule | MO | MO visualised | Symmetry |
| Cis-butadiene | LUMO | 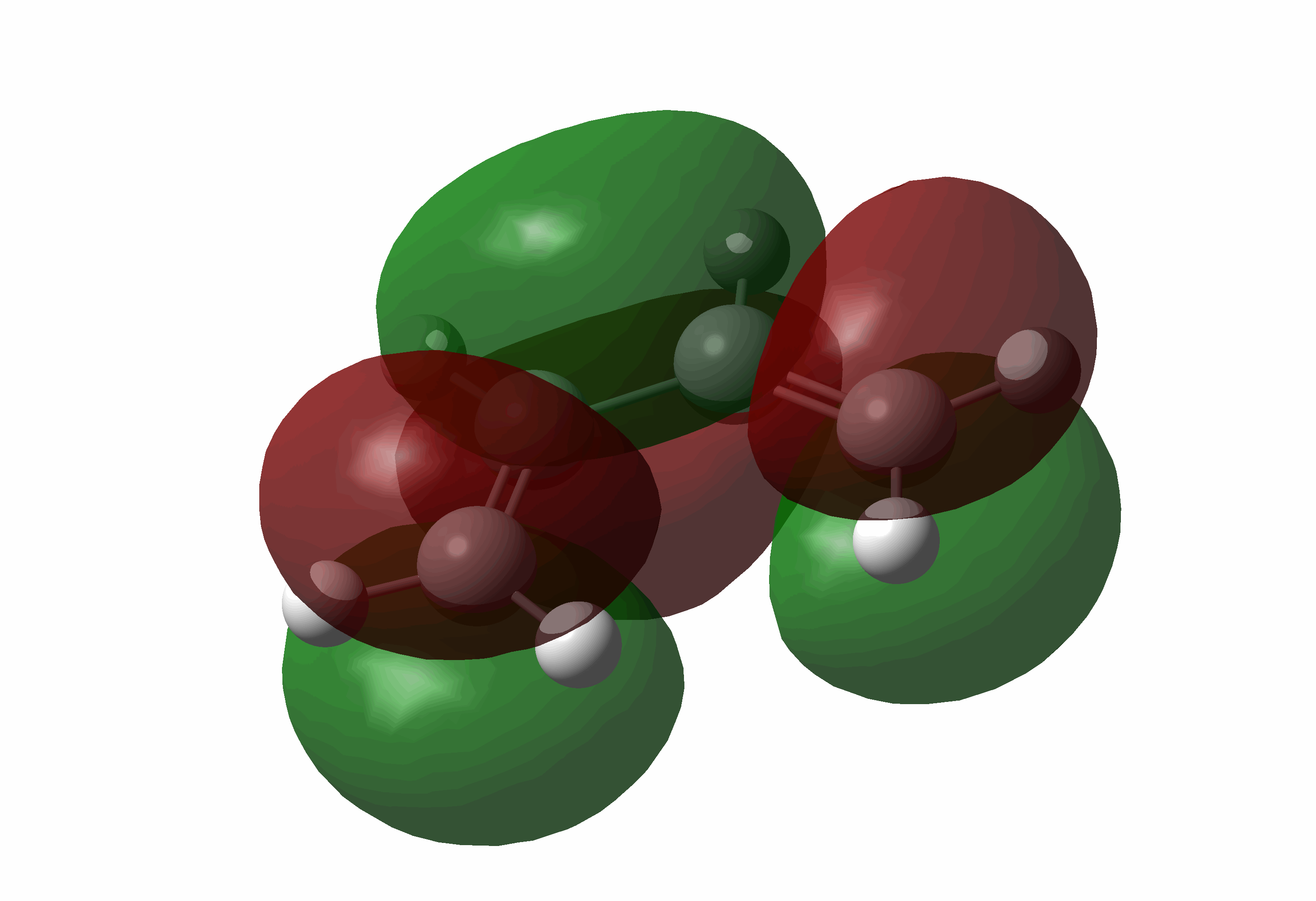 |
Symmetric |
| Cis-butadiene | HOMO | 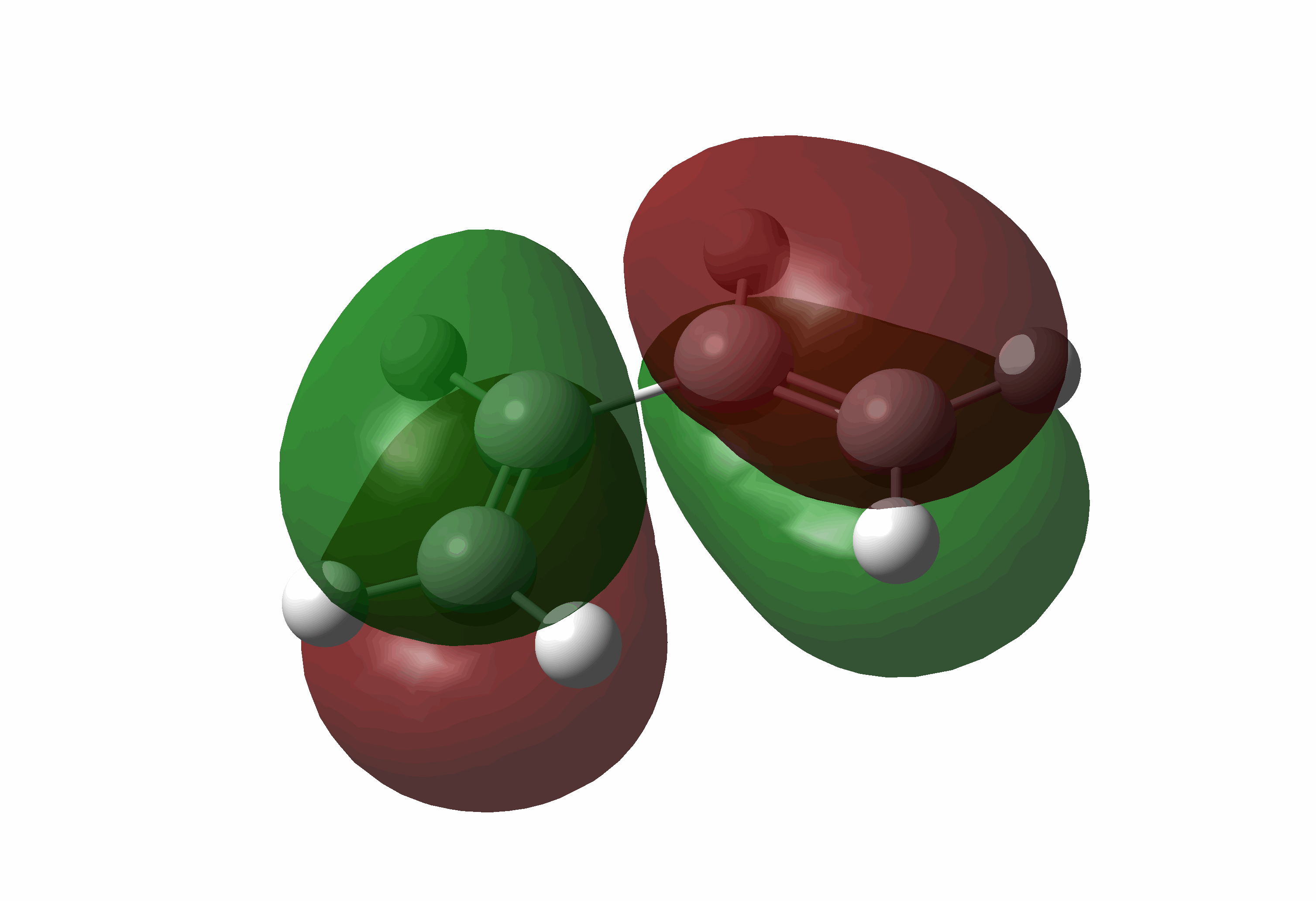 |
Anti-symmetric |
| Ethylene | LUMO |  |
Anti-symmetric |
| Ethylene | HOMO |  |
Symmetric |
The two molecules were then combined and using the bond fixing method, the transition state was determined. DOI:10042/to-7282
The following MO's were achieved:

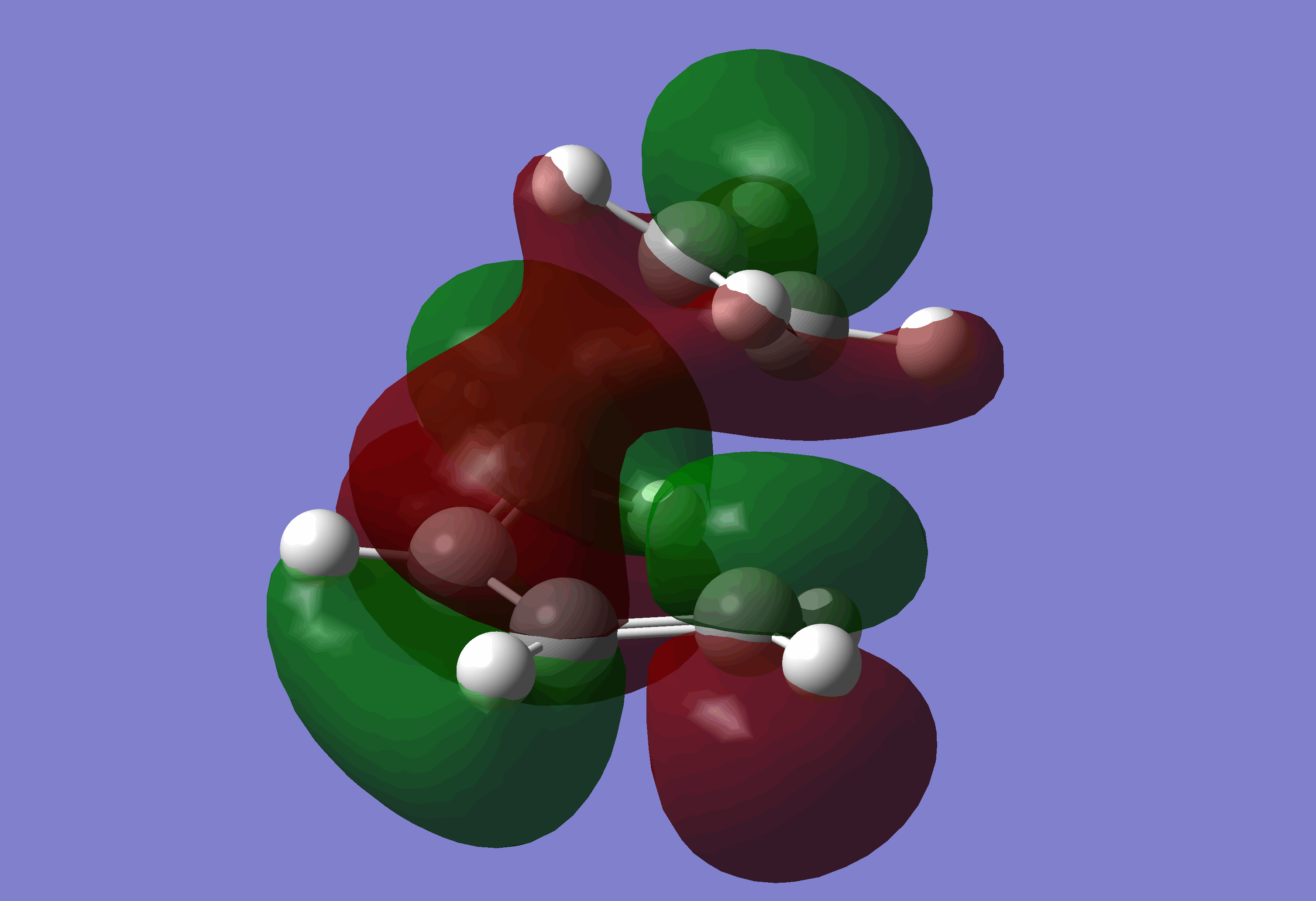
As you can see the HOMO is anti-symmetric and LUMO is symmetric, which agrees with the discussion in the given task.
The TS was confirmed by running a IRC calculation in both directions, and the above TS was indeed the maximum on the reaction coordinate graph.
The HOMO of the TS is the combination of the HOMO of the cis-butadiene and the LUMO of the ethylene, and the LUMO is the opposite.
The C-C separation at the new bonding sites was noted to be 2.26354 and 2.26297 Å. The double bonds on the cis-butadiene are 1.38880 Å, single C-C bond is 1.40798 Å and the ethylene double bond is 1.39274 Å.
Diels Alder
TS1 optimisation fixed
TS2 optimisation
TS1 failed final opt
TS2 failed final opt
<jmol> <jmolAppletButton>
In this exercise, I was unable to get a fully optimised transition structure. tried various methods, but the best was the bond fixing. The above are the structure of the exo and endo TS's. But on final optimisations, the structure would distort unrealistically.
