Rep:Mod:bl2518
Ammonia
Basic information
Molecule name = Ammonia
Calculation Type = FREQ
Calculation Method = RB3LYP
Basis Set = 6-31G(d,p)
E(RB3LYP) = -56.55776873 a.u.
RMS Gradient Norm = 0.00000485 a.u.
Point Group = C3V
optimised N-H bond distance = 1.018Å
optimised H-N-H bond angle = 106°
"Item" table
Item Value Threshold Converged?
Maximum Force 0.000006 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000014 0.001800 YES
RMS Displacement 0.000009 0.001200 YES
Predicted change in Energy=-1.141620D-10
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.018 -DE/DX = 0.0 !
! R2 R(1,3) 1.018 -DE/DX = 0.0 !
! R3 R(1,4) 1.018 -DE/DX = 0.0 !
! A1 A(2,1,3) 105.7446 -DE/DX = 0.0 !
! A2 A(2,1,4) 105.7446 -DE/DX = 0.0 !
! A3 A(3,1,4) 105.7446 -DE/DX = 0.0 !
! D1 D(2,1,4,3) -111.8637 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
GradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGrad
Jmol
ammonia molecule |
The optimisation file is liked to here
Vibrations analysis
| wavenumber/cm-1 | symmetry | intensity/arbitrary units | motion |
|---|---|---|---|
| 1089 | A1 | 145 | 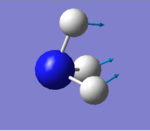
|
| 1694 | E | 14 | 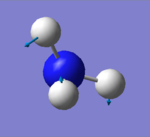
|
| 3461 | A1 | 1 | 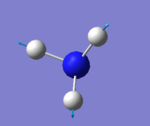
|
| 3590 | E | 0 | 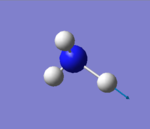
|
| 3590 | E | 0 | 
|
| 1694 | E | 14 | 
|
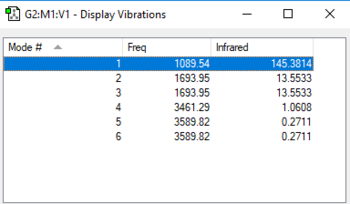
Questions:
how many modes do you expect from the 3N-6 rule? 6 modes
which modes are degenerate (ie have the same energy)? mode2&3 and mode5&6
which modes are "bending" vibrations and which are "bond stretch" vibrations? bending vibrations: mode1,2&3 bond stretching vibrations: mode4,5&6
which mode is highly symmetric? mode4
one mode is known as the "umbrella" mode, which one is this? mode1
how many bands would you expect to see in an experimental spectrum of gaseous ammonia? 2 bands
Charge analysis
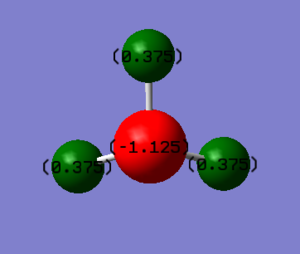
charge on the N-atom: -1.125
charge on the H-atoms: 0.375
Nitrogen should be negatively charged, but hydrogen should be positively charged. Because nitrogen has larger electronegativity.
Nitrogen
Basic information
Molecule name = Nitrogen
Calculation Type = FREQ
Calculation Method = RB3LYP
Basis Set = 6-31G(d,p)
E(RB3LYP) = -109.52359111 a.u.
RMS Gradient Norm = 0.02473091 a.u.
Dipole Moment = 0.0000 Debye
Point Group = D∞H
optimised N-N bond distance = 1.092Å
"Item" table
Item Value Threshold Converged?
Maximum Force 0.000001 0.000450 YES
RMS Force 0.000001 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000000 0.001200 YES
Predicted change in Energy=-3.400988D-13
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.1055 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
GradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGrad
Jmol
nitrogen molecule |
The optimisation file is liked to here
Vibrations analysis
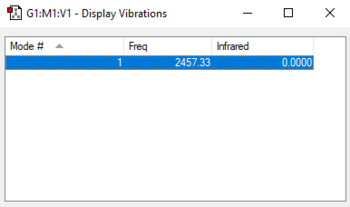
| wavenumber/cm-1 | symmetry | intensity/arbitrary units | motion |
|---|---|---|---|
| 2457 | SGG | 0 | 
|
Charge analysis
charge on the N-atom: 0
Nitrogen is a neutral symmetric molecule.
Transition metal complex
I found (bis(2-(dicyclohexylphosphino)phenyl)(methyl)silyl)-dinitrogen-(diphenyl(methyl)phosphino)-cobalt structure VEJSOV and the N-N distance is 1.116(5)Å
The computational N-N bond distance is close to the distance in the complex structure, the difference is only 0.0265Å. This is because the programme has the basis set, 6-31G(d,p) which only has a medium level accuracy, the programme cannot give the exact value and have rounding error. When these two bond distances round two 1 dp, they have the exact same value. The complex bond distance is longer and weaker, so the electron density between N atoms is lower. To clarify, N atom makes bond to the transition metal, electron density diffuses.
Hydrogen
Basic information
Molecule name = Hydrogen
Calculation Type = FREQ
Calculation Method = RB3LYP
Basis Set = 6-31G(d,p)
E(RB3LYP) = -1.17853936 a.u.
RMS Gradient Norm = 0.09719500 a.u.
Dipole Moment = 0.0000 Debye
Point Group = D∞H
optimised H-H bond distance = 0.743Å
"Item" table
Item Value Threshold Converged?
Maximum Force 0.000000 0.000450 YES
RMS Force 0.000000 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000001 0.001200 YES
Predicted change in Energy=-1.164080D-13
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 0.7428 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
GradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGrad
Jmol
hydrogen molecule |
The optimisation file is liked to here
Vibrations analysis
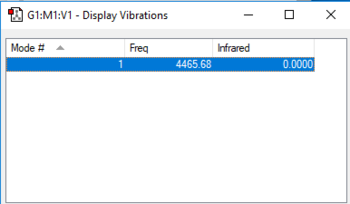
| wavenumber/cm-1 | symmetry | intensity/arbitrary units | motion |
|---|---|---|---|
| 4466 | SGG | 0 | 
|
Charge analysis
charge on the H-atom: 0
Hydrogen is a neutral symmetric molecule.
Energy of Haber process
E(NH3)= -56.5577687a.u.
2*E(NH3)= -113.1155374a.u.
E(N2)= -109.5235911a.u.
E(H2)= -1.1785394a.u.
3*E(H2)= -3.35356182a.u.
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -0.0563281a.u. = -147.9kJ/mol
Ammonia is more stable, because the enthalpy change of the reaction is negative. The reaction is exothermic, energy reduces and is given off.
Oxygen
Basic information
Molecule name = Oxygen
Calculation Type = FREQ
Calculation Method = RB3LYP
Basis Set = 6-31G(d,p)
E(RB3LYP) = -150.25250603 a.u.
RMS Gradient Norm = 0.05905207 a.u.
Dipole Moment = 0.0000 Debye
Point Group = D∞H
optimised O-O bond distance = 1.162Å
"Item" table
Item Value Threshold Converged?
Maximum Force 0.000130 0.000450 YES
RMS Force 0.000130 0.000300 YES
Maximum Displacement 0.000080 0.001800 YES
RMS Displacement 0.000113 0.001200 YES
Predicted change in Energy=-1.033738D-08
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.216 -DE/DX = -0.0001 !
--------------------------------------------------------------------------------
GradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGrad
Jmol
hydrogen molecule |
The optimisation file is liked to here
Vibrations analysis
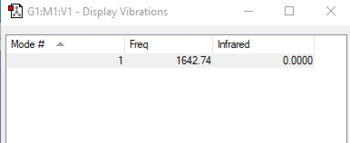
| wavenumber/cm-1 | symmetry | intensity/arbitrary units | motion |
|---|---|---|---|
| 1643 | SGG | 0 | 
|
Charge analysis
charge on the O-atom: 0
Oxygen is a neutral symmetric molecule.
MOs analysis
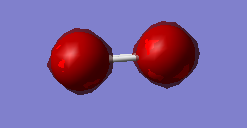
Fig.1 MO is a bonding combination of the two 1s core AOs on each oxygen atom. It is the lowest energy (-19.30736a.u.) MO of oxygen.
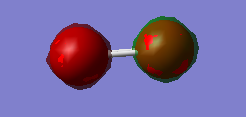
Fig.2 MO is an antibonding combination of the two 1s core AOs on each oxygen atom. The two 1s AOs have different phases, so it is an antibonding. Its MO energy is -19.30712a.u. which is also low enough. Although its energy is still higher than 1s bonding MO.
These two MOs are deep in energy when they are compared with MOs from valence shell. 1s AOs are tightly bounded by the nucleus, so they are hard to overlap with each other. Thus, they are rarely involved with chemical bonding.
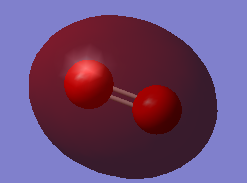
Fig.3 MO is a bonding combination of the two 2s valence AOs on each oxygen atom. It has much higher energy (-1.27663a.u.) than 1s bonding orbital. Because 2s orbital energy is higher than 1s and 2s electron is not strongly attracted by nucleus.
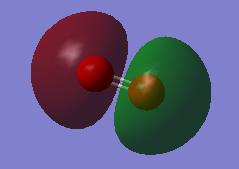
Fig.4 MO is an antibonding combination of the two 2s valence AOs on each oxygen atom. The MO energy is -0.79821a.u.
As shown in the graph, the MO is more extensive. They are very involved in the chemical bonding. The energy difference (0.47842a.u.) between 2s bonding and antibonding is greater than energy difference (0.00024a.u.) of 1s. This is due to better overlap of valence 2s AOs.

Fig.5 MO is the pi bonding of the two 2p AOs on each oxygen atom. They are perpendicular to the oxygen bond, and overlap side by side. It is also involved in the chemical bonding. Its energy is -0.51a.u. Actually, there are in total four 2p AOs overlap to form two degenerate pi MOs. However, their energy is higher than 2s-2s MO.
Marking
Note: All grades and comments are provisional and subject to change until your grades are officially returned via blackboard. Please do not contact anyone about anything to do with the marking of this lab until you have received your grade from blackboard.
Wiki structure and presentation 1/1
Is your wiki page clear and easy to follow, with consistent formatting?
YES
Do you effectively use tables, figures and subheadings to communicate your work?
YES
NH3 1/1
Have you completed the calculation and given a link to the file?
YES
Have you included summary and item tables in your wiki?
YES
Have you included a 3d jmol file or an image of the finished structure?
YES
Have you included the bond lengths and angles asked for?
YES
Have you included the “display vibrations” table?
YES
Have you added a table to your wiki listing the wavenumber and intensity of each vibration?
YES
Did you do the optional extra of adding images of the vibrations?
YES
Have you included answers to the questions about vibrations and charges in the lab script?
YES - all correct, well done.
N2 and H2 0/0.5
Have you completed the calculations and included all relevant information? (summary, item table, structural information, jmol image, vibrations and charges)
YES
You have given an incorrect N-N bond distance because you accidently measured the distance from the unoptimised structure rather than the optimised stucture. In the optimised parameter table in your wiki you can see the correct value!
Crystal structure comparison 0.5/0.5
Have you included a link to a structure from the CCDC that includes a coordinated N2 or H2 molecule?
YES
Have you compared your optimised bond distance to the crystal structure bond distance?
YES
Haber-Bosch reaction energy calculation 1/1
Have you correctly calculated the energies asked for? ΔE=2*E(NH3)-[E(N2)+3*E(H2)]
YES
Have you reported your answers to the correct number of decimal places?
YES
Do your energies have the correct +/- sign?
YES
Have you answered the question, Identify which is more stable the gaseous reactants or the ammonia product?
YES
Your choice of small molecule 4/5
Have you completed the calculation and included all relevant information?
YES
Have you added information about MOs and charges on atoms?
YES - Good explanations well done! You could be a little more careful with your grammar to help readers better understand the explanations.
Independence 0/1
If you have finished everything else and have spare time in the lab you could: Check one of your results against the literature, or Do an extra calculation on another small molecule, or Do some deeper analysis on your results so far
