Rep:Mod:bgasuki2
Introduction
In this module, the transition structures in larger molecules such as Cope rearrangement and Diels Alder cycloaddition reactions will be studied through solving the Schrodinger equation numerically and
we use molecular orbital-based methods, numerically solving the Schrodinger equation, and locating transition structures based on the local shape of a potential energy surface. As well as showing what transition structures look like, reaction paths and barrier heights can also be calculated.
The Cope Rearrangement
The Cope Rearrangement is a pericyclic [3,3]-sigmatropic rearrangement reaction which involves a breaking and forming of a σ-bond in a concerted fashion as shown in Fig. 1.1.

After years of controversy, recent research[1] has confirmed that the reaction undergoes a concerted mechanism with two possible geometries for its transition state: the chair or the boat conformation. In this module, both the 1,5-hexadiene reactant and product, the chair and boat transition structures, will be studied through means of different optimisation methods.
Optimisation of Reactant and Product
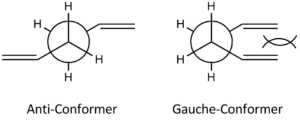
In the structure of 1,5-hexadiene molecule, two C=C double bonds are located at both ends of the chain and are linked by 4 C-C single bonds. These singly bonded carbon atoms are able to rotate freely about the σ-bond, generating many possible conformers for both the reactant and the product. The two main conformers that will be discussed are the anti-conformer and the gauche-conformer. To determine the structure of each conformer, one common way of picturing these two is to generate a staggered conformation in the Newman projection as shown below.
 |
 |

For anti-conformation, the two alkene groups are anti-periplanar to each other, with the dihedral angle between carbon C2-C3-C4-C5 is 180o. For gauche-conformation, the two alkene groups are syn-clinical (or Gauche) to each other with the dihedral angle between carbon C2-C3-C4-C5 is 60o.
A) Optimisation of anti-conformer
The anti-1,5-hexadiene conformer was drawn in Gaussview with the dihedral angle between C2 to C5 is 180o. This structure was then cleaned and optimised with the following input command:
Job type: optimisation
The method: Hartree-Fock
The basis set: 3-21G
| Conformer | Energy (a.u.) | Point Group | Output Link | ||||
|---|---|---|---|---|---|---|---|
| anti-conformer |
|
-231.69253527 | Ci | Anti Conformer | |||
By comparing both the energy, the point group and the structure, this anti-conformer corresponds to the anti 2 structure in Appendix 1 of Module 3.
B) Optimisation of gauche-conformer
The gauche-1,5-hexadiene conformer was drawn in Gaussview with the dihedral angle between C2 to C5 is 60o. This structure was then cleaned and optimised with the following input command:
Job type: optimisation
The method: Hartree-Fock
The basis set: 3-21G
| Conformer | Energy (a.u.) | Point Group | Output Link | ||||
|---|---|---|---|---|---|---|---|
| gauche-conformer |
|
-231.69166701 | C2 | Gauche Conformer | |||
Also by comparison, this conformer corresponds to Gauche 2 conformer as listed in Appendix 1 of Module 3.
C) Hypothesis of Lowest Energy Conformer
From the Newman projection above, the Gauche conformer is expected to experience greater steric hindrance and hence will be less stabilised. Therefore the anti conformer is expected to be the lower energy conformer. However, there are still different possible structures of anti conformers and in order to predict the lowest energy conformer, the structure is altered manually and optimised with the same input command:
Job type: optimisation
The method: Hartree-Fock
The basis set: 3-21G
The results of different conformers are summarised and tabulated in part D).
D) Identification of Optimised Structure
Different conformers are manually adjusted by altering the 3 main dihedral angles between the carbons, with the numbering of carbon atoms correspond to that as labelled in Fig.1.4. The dihedral angles listed below are the angles that were set before optimisation.
| Conformer | Corresponding structure in Appendix 1 | Dihedral Angle between C1 to C4 | Dihedral Angle between C2 to C5 | Dihedral Angle between C3 to C6 | Energy (a.u.) | Point Group | Output Link | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anti conformer 1 |
|
anti 2 | 120o | 180o | 120o | -231.69253527 | Ci | Anti Conformer 1 | |||
| Anti conformer 2 |
|
anti 3 | 0o | 180o | 0o | -231.68907050 | C2h | Anti Conformer 2 | |||
| Anti conformer 3 |
|
anti 4 | 60o | 180o | 120o | -231.68937804 | C1 | Anti Conformer 3 | |||
| gauche conformer 1 |
|
gauche 2 | 180o | 60o | 180o | -231.69166701 | C2 | Gauche Conformer 1 | |||
| gauche conformer 2 |
|
gauche 3 | 120o | 60o | 180o | -231.69266110 | C1 | Gauche Conformer 2 | |||
| gauche conformer 3 |
|
gauche 1 | 0o | 60o | 0o | -231.68771613 | C2 | Gauche Conformer 3 | |||
From the information above, it is surprise to discover that the lowest energy conformer is the Gauche 1 conformer (energy of -231.68771613 Hartree a.u.) instead of an anti conformer as predicted. When considering the relative stability of a molecule, stereo-electronic factor is usually considered together with the sterics. Since the Gauche conformer is the most stabilised one, the favourable electronic interaction in this conformation overrides the strain arises from sterics.
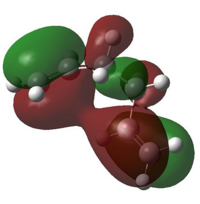
To have a better understanding of the electronic interaction of Gauche 1 conformer, its frontier molecular orbitals were simulated from its checkpoint file and in particular the HOMO and LUMO of Gauche 1 were pictured:
 |
 |
In the HOMO of Gauche 1 molecule, the two alkene bonds are orientated in such a way that the π cloud orbitals of both C=C bonds are in phase with each other, allowing favourable π- π interactions and therefore stabilised the molecule. Similar interactions can also be found in the LUMO, where the antibonding orbitals of both C=C*bonds are aligned in the correct orientation and allowed favourable orbital overlap between the two alkene groups, hence stabilising the molecule. This stabilising interaction cannot be found in anti-conformers as the two alkene groups are pointed away from each other, hindering the possibility of orbital overlap. These favourable orbital interactions in Gauche 1 conformer outweighed the destabilisation arose from sterics clashes and is the lowest energy conformer.
E) Optimisation of anti 2 Conformer
The required anti 2 conformer was optimised previously in the initial optimisation for anti conformer at HF/3-21G level as shown above with point group of Ci symmetry.
F) Comparison between Optimisation at HF/3-21G and DFT-B3LYP/6-31G Level
DFT optimisation method is another optimisation method with higher accuracy and was applied to the anti 2 conformer with the following input:
Job type: optimisation
The method: DFT – B3LYP
The basis set: 6-31G
| Basis Set | Output Logfile | Jmol modelling | Point Group | Energy/ Hartree a.u. | Energy / Kcal mol-1 |
| HF/3-21G | HF/3-21G opt. | 'XML error: > required at line 5 | C1 | -231.69254 | -608308.75 |
| DFT-B3YLP/6-31G | DFT-B3LYP /6-31G opt. | ' | C1 | -234.55970 | -615836.48 |
| Energy Difference | - | - | -2.86716 | -7527.73 |
| Basis Set | Dihedral angle (C1-C4)/o | Dihedral angle(C2-C5)/o | Dihedral angle(C3-C6)/o | C=C bond length/Å | C2-C3/ C4-C5 bond length/ Å | C3-C4 bond length/ Å |
| HF/3-21G | 114.63 | 179.96 | 114.63 | 1.3162 | 1.5089 | 1.5530 |
| DFT-B3YLP/6-31G | 118.71 | 180.00 | 118.71 | 1.3383 | 1.5072 | 1.5550 |
From the 3D model, there is no visible difference between the two optimised anti-2 conformer. The physical data for both were compatible and has one noticeable deviation on the diherdral angle between C1-C4 and C3-C6. This value produced for optimising at HF/3-21G level is 114.63o whereas that for optimising at DFT-B£YLP/6-31G level is 118.71o. The C=C bond length of ~1.32 Å and C-C bond length of ~1.51 Å /1.55 Å are in good agreement with the literature and therefore both optimisation method yields pretty accurate result for 1,5hexadiene molecule. ?????ENERGY DISCUSSION??????????
G) ????
- ↑ Olaf Wiest, Kersey A. Black, K. N. Houk, J. Am. Chem. Soc., 1994, 116(22), pp 10336-10337.DOI:10.1021/ja0010a078
