Rep:Mod:bb406
Birendra Balakrishnan
Structure & Spectroscopy
The Hydrogenation of Cyclopentadiene Dimer
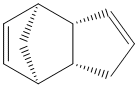
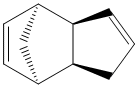
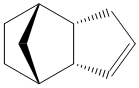
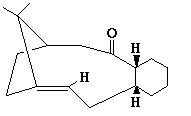
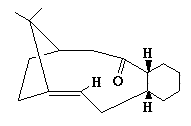
An analysis was carried out on the energy contribution of strecthing (str), bending (bnd), torsion (tor), van der Waals (vdw) and H-bonding (H-bond) on the endo (Fig. 1) and exo (Fig. 2) dimers of cyclopentadiene. Further investigation was taken into the hydrogenated forms of the endo dimer (Fig. 3) (Fig. 4).
When cyclopentadiene dimerises it preferentially produces the endo dimer over the exo dimer. Subsequent hydrogenation of the endo dimer initially gives one of the dihydro derivatives. On continued hydrogenation the tetrahydro derivative is formed.
|
Exo Dimer (1) |
Endo Dimer (2) |
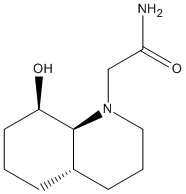
Hydrogenated Endo Dimer (3) |
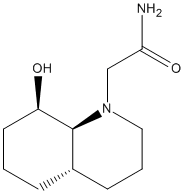
Hydrogenated Endo Dimer (4) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
Using the MM2 modelling technique, the geometries and energies for all four molecules were calculated.
|
Energies (kcal/mol) |
Exo Dimer (1) |
Endo Dimer (2) |
Hydrogenated Endo Dimer (3) |
Hydrogenated Endo Dimer (4) |
|---|---|---|---|---|
|
Stretch |
1.9962 |
1.9757 |
2.0151 |
1.9158 |
|
Bend |
21.5727 |
21.6608 |
19.9050 |
15.4671 |
|
Str-Bend |
-0.7501 |
-0.7374 |
-0.6532 |
-0.4740 |
|
Torsion |
8.4461 |
10.6446 |
13.2630 |
13.6539 |
|
Non 1,4 VdwW |
-1.9836 |
-2.2530 |
-1.3458 |
-1.1543 |
|
1,4 VdW |
5.3674 |
5.6964 |
6.8202 |
5.7185 |
|
Dipole-Dipole |
0.3770 |
0.4557 |
0.1638 |
0.1403 |
|
TOTAL |
35.0258 |
37.4428 |
40.1681 |
35.2672 |
As the lowest in energy, the exo dimer is the most thermodynamically stable. As such it would be expected to be the major product. However, as the endo dimer dominates it is a fair to assume this dimer is the kinetic product. The reaction is kinetically controlled with the endo intermediate formed during the reaction being lower in energy than the exo intermediate.
The hydrogenated endo dimer ,4, is lower in energy than the other hydrogenated product ,3. The hydrogenation of dicyclopentadiene is a thermodynamically controlled process. Therefore, the more thermodynamically stable product predominates. The rate of reaction is dependent on the rate of diffusion of hydrogen onto the reagent molecule. Molecule 4 allows faster diffusion of hydrogen than its counterpart.
On closer comparison of the relative contributions of total energy by different modes of motion and interactions, it became apparent that bending strain in molecule 4 was lower than that in molecule 3 (approximately 48% lower). This is due to the double bond in molecule 4 being under less strain. All other interactions were relatively comparable.
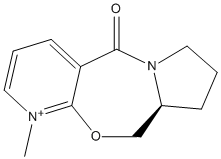
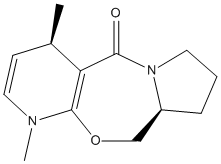
Stereochemistry of Nucleophilic additions to a pyridinium ring (NAD+ analogue)
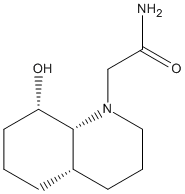
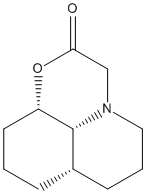
Alkylation of prolinol
An optically active derivative was reacted with a Grignard reagent, methyl magnesium iodide, to alkylate the pyridine ring on the fourth position.
|
Reagent (5) |
Product (6) |
||||||
|---|---|---|---|---|---|---|---|
|
|
Using the MM2 modelling technique, the geometries and energies were calculated.
|
Energies (kcal/mol) |
Reagent (5) |
Product (6) |
|---|---|---|
|
Stretch |
1.1863 |
1.4771 |
|
Bend |
11.3802 |
14.5694 |
|
Str-Bend |
0.0495 |
0.1630 |
|
Torsion |
5.0797 |
5.4644 |
|
Non 1,4 VdwW |
-2.0057 |
-2.0224 |
|
1,4 VdW |
11.8968 |
13.3054 |
|
Dipole-Dipole |
-3.9662 |
-4.0628 |
|
Charge-Dipole |
2.7003 |
--- |
|
TOTAL |
26.3208 |
28.8942 |
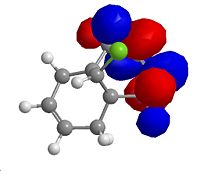
The carbonyl group, of the reagent, is not coplanar with the aromatic ring system. The carbonyl group is angled away from the plane of the pyridine ring, anti to the chiral hydrogen. The expected dihedral angle in a perfectly planar system is 0, whereas in this molecule the angle is 24.3975.
The product is higher in energy than that of the reagent. This is possibly due to the loss of aromaticity from the pyridine system.
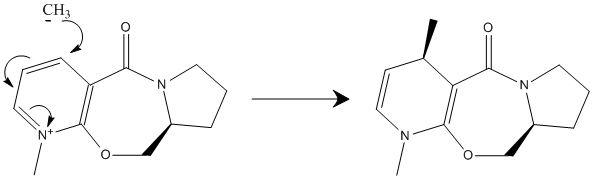
Mechanistic Control
Methyl magnesium iodide reacts as a methyl carbanion and magnesium iodide cation.
The reaction is stereo-specific as the methyl carbanion is delivered to the top face of the molecule. This is a result of coordination of the magnesium iodide cation with the amide oxygen atom. Conjugate delivery of the methyl ligand from magnesium to the carbon attacked would generate the magnesium enolate.
Improvements can be made to the model if factors such as activation barriers and the energies of transition states were included. These aspects of the reaction would provide more information on which to provide a more comprehensive analysis. Furthermore, analysis on thermodynamic or kinetic control can be made.
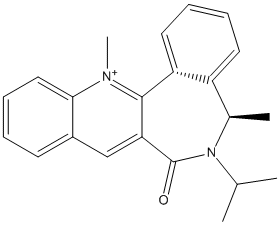
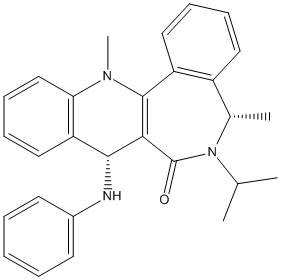
Nucleophilic Addition of NHPh
This is another example of stereo control of a reaction.
|
Reagent (7) |
Product (8) |
||||||
|---|---|---|---|---|---|---|---|
|
|
Using the MM2 modelling technique, the geometries and energies were calculated.
|
Energies (kcal/mol) |
Reagent (7) |
Product (8) |
|---|---|---|
|
Stretch |
1.7056 |
1.8980 |
|
Bend |
7.2918 |
13.5500 |
|
Str-Bend |
0.3846 |
0.6436 |
|
Torsion |
-5.7456 |
-11.6096 |
|
Non 1,4 VdwW |
-1.1902 |
-2.3155 |
|
1,4 VdW |
17.4066 |
20.6211 |
|
Dipole-Dipole |
-4.7586 |
-4.8676 |
|
Charge-Dipole |
2.5603 |
--- |
|
TOTAL |
17.6545 |
17.9200 |
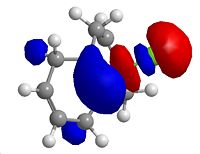
The amide carbonyl group of the reagent points down from the plane of the aromatic system. The dihedral angle between this group and the ring is 42.5706.
The NHPh group attacks from the top face of the molecule in order to avoid electronic repulsion between the attacking phenyl group and the amide carbonyl. To this end, the NHPh group attacks from the top face and points upward, anti to the carbonyl.
Stereochemistry & Reactivity of an Intermediate in the synthesis of Taxol
Taxol is an important drug in the treatment of ovarian cancer. Proposed by Paquette, the initial synthesis of the intermediate has the carbonyl group pointing either up or down.
While standing, the intermediate isomerises to the alternative carbonyl isomer. This type of isomerism is known as atropisomerism.
|
Intermediate (10) |
Intermediate (11) |
||||||
|---|---|---|---|---|---|---|---|
|
|
Using the MM2 modelling technique the relative energies were calculated.
|
Energies (kcal/mol) |
Intermediate (10) |
Intermediate (11) |
|---|---|---|
|
Stretch |
2.8191 |
2.6015 |
|
Bend |
16.4262 |
13.5803 |
|
Str-Bend |
0.4568 |
0.3410 |
|
Torsion |
21.3581 |
21.0663 |
|
Non 1,4 VdwW |
-0.8530 |
-0.8134 |
|
1,4 VdW |
14.0263 |
13.5761 |
|
Dipole-Dipole |
0.1366 |
0.2598 |
|
TOTAL |
54.3702 |
50.6116 |
Intermediate 11 is energetically more stable than Intermediate 10. However, the difference in energies is small providing favourable conditions for the isomerism between the two forms. Intermediate 11 is a hyperstable olefin due to the larger ring size and the stability provided by the caged structure of the olefin and the strain of the parent polycycloalkane.
Through minor adjustments, the energy of the intermediate was lowered. However, the energy minimisation process would often revert the structure to a previous iteration unavoidably. There are substantial difficulties in using such a modelling technique.
Room Temperature Hydrolysis of a Peptide
By using careful conformational anaylsis, it is possible to understand the mechanistic control of a reaction by two isomers. The two isomers are structurally very similar, but have very different rates of reaction.
Firstly, anaylsis was carried out on the cis isomer.
|
Reagent 13 (Axial) |
Reagent 13 (Equitorial) |
Product 13 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
Using the MM2 modelling technique, the geometries and energies for all four molecules were calculated.
|
Energies (kcal/mol) |
Reagent 13 (Axial) |
Reagent 13 (Equitorial) |
Product 13 |
|---|---|---|---|
|
Stretch |
1.4093 |
1.9333 |
1.2373 |
|
Bend |
7.8455 |
5.4342 |
8.0489 |
|
Str-Bend |
0.5890 |
0.5900 |
0.6174 |
|
Torsion |
11.5518 |
9.0388 |
6.1829 |
|
Non 1,4 VdwW |
-8.1596 |
-4.6424 |
-3.3109 |
|
1,4 VdW |
10.4828 |
10.1843 |
14.9312 |
|
Dipole-Dipole |
-4.4289 |
-3.0162 |
4.2989 |
|
TOTAL |
19.2898 |
19.5219 |
32.0056 |
The trans isomer was then studied.
|
Reagent 14 (Axial) |
Reagent 14 (Equitorial) |
Product 14 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
Using the MM2 modelling technique, the geometries and energies for all four molecules were calculated.
|
Energies (kcal/mol) |
Reagent 14 (Axial) |
Reagent 14 (Equitorial) |
Product 14 |
|---|---|---|---|
|
Stretch |
1.5415 |
1.4521 |
1.2525 |
|
Bend |
5.5936 |
3.6417 |
6.4188 |
|
Str-Bend |
0.5768 |
0.4964 |
0.5666 |
|
Torsion |
9.9304 |
8.0389 |
4.1882 |
|
Non 1,4 VdwW |
-6.5075 |
-7.6863 |
-3.5266 |
|
1,4 VdW |
9.7592 |
9.9793 |
15.2661 |
|
Dipole-Dipole |
-1.5419 |
-6.1448 |
4.5363 |
|
TOTAL |
19.3521 |
9.7772 |
28.7019 |
Mechanistic Control
In order to react successfully, the hydroxy oxygen has to approach the carbonyl group in a strict orientation. A successful hydrolysis needs the oxygen lone pair to have the best overlap with the C=O π* orbital. This involves approaching in line with the C=O bond and at an elevation of 108 degrees.
The cis isomer has two further isomers, where the group attached to the nitrogen is either axial or equitorial to the ring. The axial isomer provides the best approach to hydrolyise the peptide group. However, energetically it is very similar to the equitorial isomer. This means that there is a very small barrier for isomeric conversion. This allows the equitorial isomer to react readily via the axial isomer.
As with the cis isomer; the trans isomer has axial and equitorial conformations. However, in this case the two differ in energy greatly. The reactive conformation is axial, though due to the special stability of trans decalin the most stable is the equitorial conformation. This conformation suffers least from diaxial interactions. The energy barrier between the conformations means that the equilibrium between the axial and equitorial conformations is strongly favoured to the equitorial.
The result is that the cis form is much more reactive than the trans form.
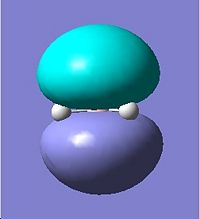
Regioselective Addition of Dichlorocarbene
|
HOMO |
HOMO -1 |
HOMO -2 |
|---|---|---|
|
LUMO |
LUMO +1 |
LUMO +2 |