Rep:Mod:awc106 module2 PROJECT2
Mini Project: Ammonia-Borane
Ammonia borane NH3BH3 is a Lewis acid-base pair molecule. NH3BH3 is also under scrutiny as a very promising molecule for the storage of hydrogen to be used as a new fuel. Ammonia-borane is 19.6% by weight hydrogen.
In this part of the report the first optimisation, molecules of NH3BH3(staggered and eclipsed) were generated in Gaussview, and then optimisations run using a basis set of 6-31G and the method used was B3LYP. The next step was to utilise a better method and basis set, the basis set used was 6-311+G(d,p) and the method used was MP2. Finally the keywords "pop=full" were included in the optimisation, and the checkpoint file examined in order to inspect the molecular orbitals of the different conformations.
Ammonia Borane
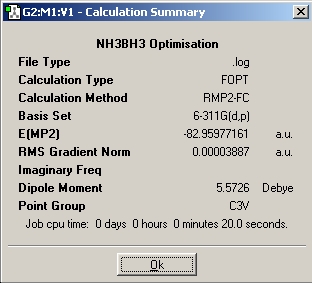
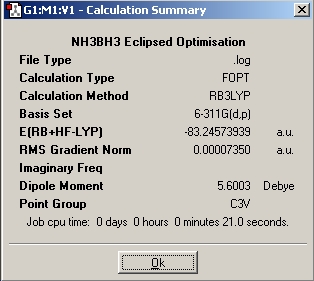
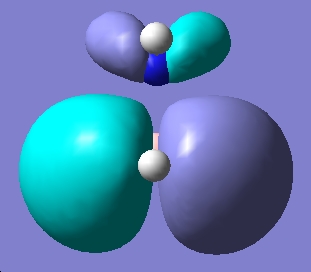
Table 1: NH3BH3 | |||||||||||
| Staggered | Eclipsed | ||||||||||
| Results | Image | HOMO | Results | Image | HOMO | ||||||
|
|
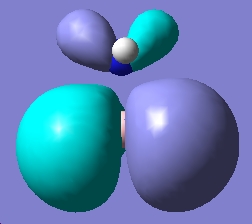
|
|
|
| ||||||
| https://www.ch.ic.ac.uk/wiki/images/e/e7/AB_1.log | https://www.ch.ic.ac.uk/wiki/images/b/b4/AB_2.log | ||||||||||
Upon examining the results obtained, we can clearly see that there is not a great difference in energy between the staggered and eclipsed conformations. We would expect however that the staggered conformation wold be lower in energy due to reduced steric hindrance of the neighboring groups.
Ethane
Ammonia-borane is isoelectronic with ethane. It is thus beneficial to compare the two molecules. The same set of calculations used for ammonia-borane were repeated for ethane.
Table 2: Ethane | |||||||||||
| Staggered | Eclipsed | ||||||||||
| Results | Image | HOMO | Results | Image | HOMO | ||||||
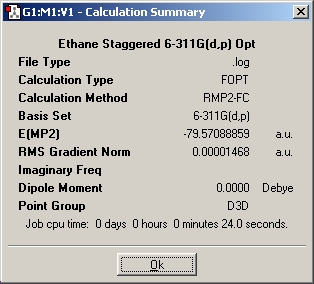
|
|
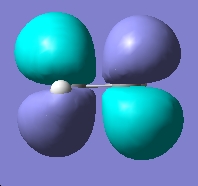
|
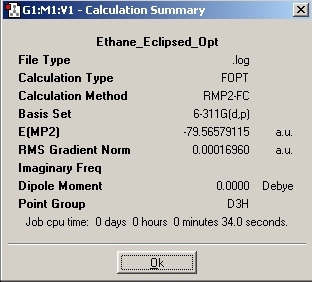
|
|
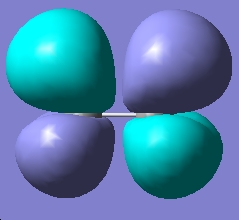
| ||||||
| https://www.ch.ic.ac.uk/wiki/images/3/38/Ethane.log | https://www.ch.ic.ac.uk/wiki/images/4/4c/Ethane_2.log | ||||||||||
Here again we can see that there is not a large energy difference between the two conformations, we know however that it is the staggered conformation which is the energy minima. We can see however, in comparison of the two sets of calculation, that the two sets of energy differences are both very small.
Reaction
It is also beneficial to determine the stability of NH3BH3 relative to its precursors. NH3BH3 is synthesised according to the following scheme:
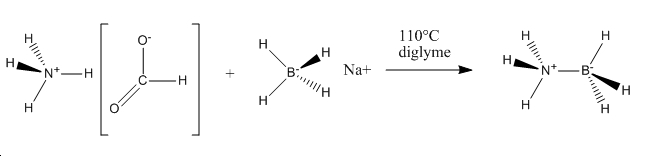
Hydrogen (H2) is then released resulting in NH3BH3. Therefore we can compare the relative energies of the reactants and products and calculate E(reactants)-E(products).
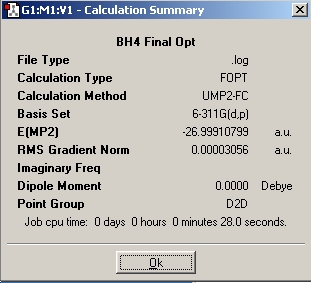
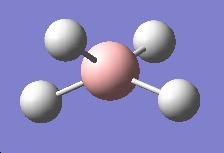
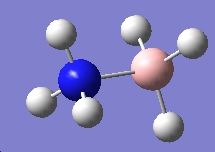
Table 3: Products and Reactants | ||
| NH4+ | BH4- | NH3BH3 |

|
|

|

|
|
|
| https://www.ch.ic.ac.uk/wiki/images/1/1e/NH4.log | https://www.ch.ic.ac.uk/wiki/images/0/0f/BH4.log | https://www.ch.ic.ac.uk/wiki/images/d/db/NH3BH3.log |
We can calculate E(reactants)-E(products) from this information. Performing this calculation, we arrive at a value of -0.79a.u. This tells us that ammonia-borane is more stable relative to its reactants, which means that it is the thermodynamic product of the reaction between sodium borohydride and ammonium chloride.
By-Products
As can be seen from the below scheme, the dehydrogenation of NH3BH3 results in several by-products.
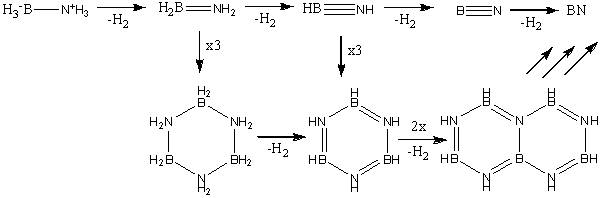
Again calculations were performed, first using a basis set of 6-31G and a method of B3LYP. The next step was to utilise a better method and basis set, the basis set used was 6-311+G(d,p) and the method used was MP2.
Table 3: Products and Reactants | ||
| Trimer | Borazine | Di-Borazine |
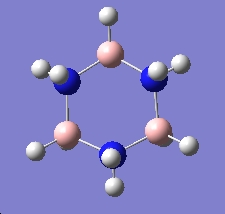
|
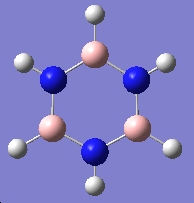
|
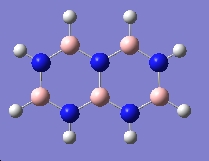
|
| -246.19 a.u. | -242.65 a.u. | -403.26 a.u. |
| C3v | D3h | C2v |
| https://www.ch.ic.ac.uk/wiki/images/3/35/Trimer.log | https://www.ch.ic.ac.uk/wiki/images/9/9c/Borazine.log | https://www.ch.ic.ac.uk/wiki/images/2/20/Di-borazine.log |
Again we can compare these with the corresponsing carbon analogues:
Table 3: Products and Reactants | ||
| Cyclohexane | Benzene | Naphthalene |
|

|
|
| -235.74 a.u. | -232.21 a.u. | -385.83 a.u. |
| D3d | D6h | D2h |
| https://www.ch.ic.ac.uk/wiki/images/d/d6/Cyclohexane.log | https://www.ch.ic.ac.uk/wiki/images/2/22/Benzene.log | https://www.ch.ic.ac.uk/wiki/images/8/84/Napthalene.log |
On first inspection we can see that the borane/nitrogen analogues appear to be the more stable molecules It also appears that the benzene and borazine molecules are less stable than their corresponding non-aromatic counter-parts. We know this to be the other way round, benzene has a far greater stability compared with cyclohexane conferred by the delocalisation of pi electrons across the whole molecule and we can infer that borazine would have a similar aromatic stability. This shows the limitations of some of the calculations that we have been performing, in that they are producing results that we know to be wrong. However we can see the napthalene type molecules have a far greater stability than the singly aromatic rings, and this is in line with what we would expect.
