Rep:Mod:awc106 module2 DAY3
Day 3: Ammonia
Initial Optimisation
In this part of the report we will be performing a similar range of calculations on the isomers of ammonia. The aim is to investigate the effect of symmetry on the structures, their energies, and the relative optimisation times. During the first optimisation, a molecule of NH3 was generated in Gaussview, and the basis set used was 6-31G and the method used was B3LYP. Then another molecule of NH3 was created and one bond was set to a length of 1.01Å, and the ignore symmetry option was invoked. FInally the nh3_b3lyp_d3h.txt provided was opened and an optimisation run. This file included a dummy atom which restricted the symmetry of the molecule.
Table 1: Initial Optimisation of NH3 | ||
| Optimisation | Results Summary | Image |
| Inital Optimisation | 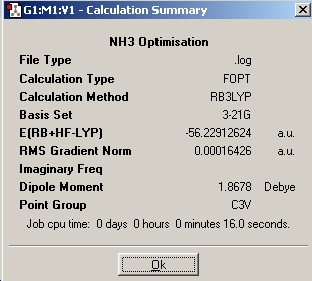
|
|
| Igmore Symmetry Optimisation | 
|
|
| Nh3_b3lyp_d3h Optimisation | 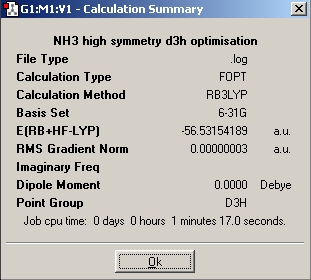
|

|
As I was unable to publish my output files on D-Space, I have linked the files below:
File:HCRAWFORD NH3 IGNORESYMMETRY OPT.jpg
Higher Basis Set
The next step is to utilise a better method and basis set, and to examine the effect it has on the time taken to perform the calculation, the final geometry and energies that are obtained. The basis set used was 6-311+G(d,p) and the method used was MP2. Next the file nh3_mp2_d3h.txt was used and an optimisation run from it. These two calculations have achieved the optimisation of the ground state of NH3 with a point group symmetry of C3v and the inversion transition state, which is planar and has D3h symmetry.
Table 1: Optimisation of NH3 With Higher Basis Set | ||
| Optimisation | Results Summary | Image |
| Inital Optimisation | 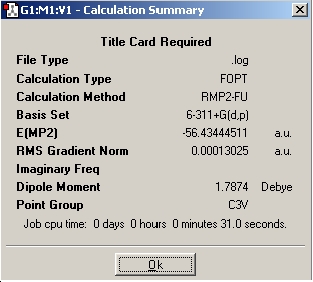
|
|
| Igmore Symmetry Optimisation | 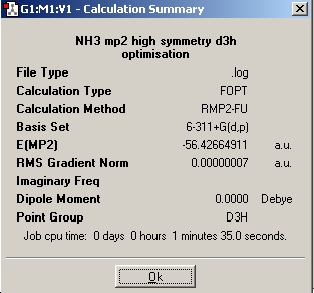
|

|
As I was unable to publish my output file on D-Space, I have linked the files below:
File:HCRAWFORD NH3 6-311 OPT.jpg
Looking at the two sets of optimisations we have just performed, we can see that the higher level MP2/6-311+G(d,p) calculations took longer to compute, approximately 15seconds longer in both cases. For the greater degree of accuracy acheived by using the higher basis set this time is acceptable, however with more complex molecules, the time difference would undoubtably be much larger.
We can also calculate ΔE=E(D3h)-E(C3v) for the two basis sets. For the B3LYP/6-31G we have an energy difference of 0.0078 a.u., and for the MP2/6-311+G(d,p) set of calculations we have -0.302 a.u. If we compare this to the experimentally determined difference of 24.3 kJ/mol, we see that whilst the higher basis set is mroe accuarate, is is not quite the same as that determined experimentally.
The Inversion Mechanism
So far we have optimised the ground state of NH3 with a point group symmetry of C3v and the inversion transition state, which is planar and has D3h symmetry. However we have not yet linked these two forms of ammonia together via a reaction pathway. Due to the shortcomings of Gaussview, a scan can not be performed. A scan varies one coordinate whilst the other coordinates optimises. In the case of ammonia, the nitrogen atom will remain stationary and the hydrogen atoms will move about it during optimisation. A scan file was provided. A scan calculation provides a graph of energy against scan step, which is a visualisation of the potential energy surface.

Under the results tab, the scan option is available. This provides a visualisation of the molecule at each scan step. Here is a summary:
Table 1: Optimisation of NH3 With Higher Basis Set | ||
| Scan Step | Image | Relative Energy |
| 1 | 
|
None |
| 12 | 
|
Energy Minima |
| 21 | 
|
Energy Maxima |
Vibrational Analysis
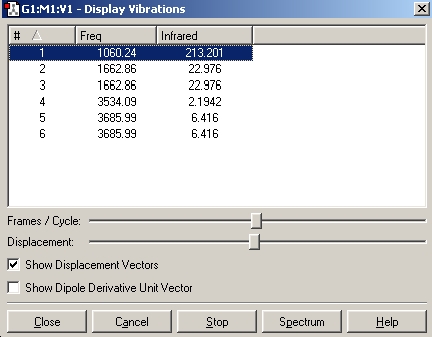
In this section we will caclulate the vibrational spectrum of NH3 of both the ground state (C3v symmetry) and the planar inversion transition state (D3h symmetry). We will be using the optimised structures from the B3LYP/6-31G calculations.
C3v

D3h
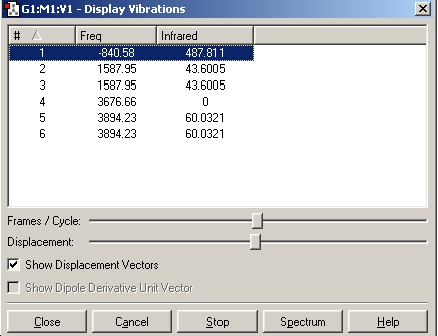
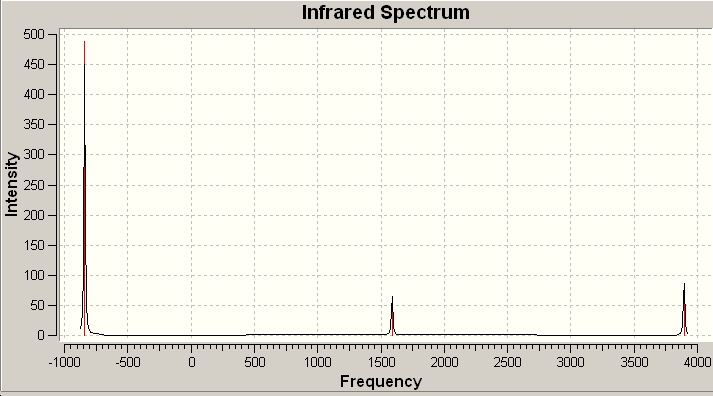
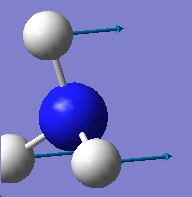
Table 1: Comparison of Vibrational Modes | |||
| Vibration Number | C3v | D3h | |
| 1 | 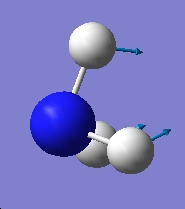
|
| |
| 2 | 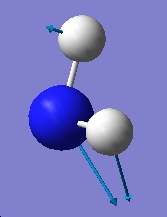
|
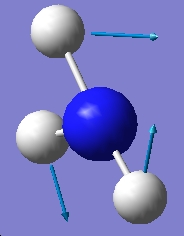
| |
| 3 | 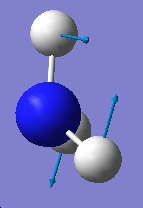
|

| |
| 4 | 
|
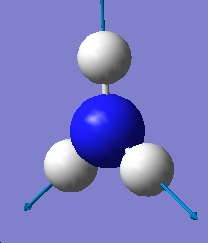
| |
| 5 | 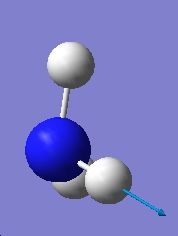
|
| |
| 6 | 
|

| |
As can be seen from the above results, in the case of the C3v structure all of the frequencies are positive, this tells us that we have the minimised structure. Compared with the D3h structure which has one negative frequency (-840.58). This tells us that thi structure is a transition state. When we examine the table of the vibrational modes, we can see that all of the vibrational modes match up to one another. We can also see that vibrational mode 1 follows the inversion pathway.
