Rep:Mod:aw106010288
Year 3:Computational Laboratory:Organic Module
Modelling using Molecular Mechanics
The Hydrogenation of Cyclopentadiene Dimer
Dimerisation
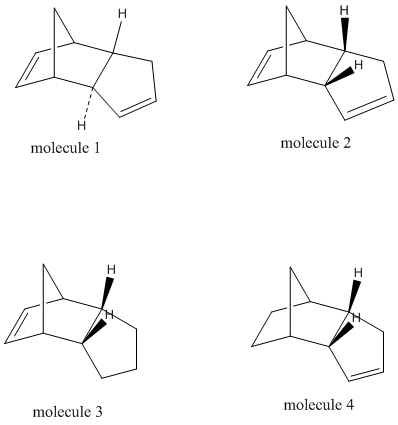
Cyclopentadiene molecules can dimerise by undergoing a diels alder reaction with one molecule acting as a dienophile and one as a diene. The two molecules can approach each other in two distinct directions and can thus produce either an endo- or exo- isomer of dicyclopentadiene. In the following exercise a series of MM2 calculations will be carried out in order to optimise the geometry and to identify the energy contributions to the total energy of each isomer. From this, suggestions can be made to whether the dimerisation process and the hydrogenation of the dimer is controlled kinetically or thermodynamically.
MM2 CALCULATIONS
|
Energy Contributions (kcal/mol) |
1
|
2
|
3
|
4
|
||||||||||||
|
Stretch |
1.2765 |
1.2541 |
1.2324 |
1.1292 |
||||||||||||
|
Bend |
20.5699 |
20.8279 |
18.8641 |
13.0405 |
||||||||||||
|
Stretch-Bend |
-0.8326 |
-0.8370 |
-0.7625 |
-0.5672 |
||||||||||||
|
Torsion |
7.6637 |
9.5168 |
12.2469 |
12.3813 |
||||||||||||
|
Non-1,4 VDW |
-1.4101 |
-1.5427 |
-1.5627 |
-1.3055 |
||||||||||||
|
1,4 VDW |
4.2363 |
4.3320 |
5.7523 |
4.4450 |
||||||||||||
|
Dipole/Dipole |
0.3779 |
0.4486 |
0.1631 |
0.1411 |
||||||||||||
|
Total Energy |
31.8817 |
33.9998 |
35.9337 |
29.2644 |
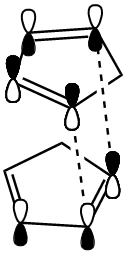
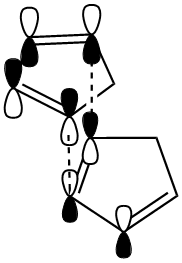
From the MM2 calculations it can be seen that the exo product has a lower total energy (-2.1181 kcal/mol) than the endo product and is thus the more thermodynamically stable of the two. The endo product however "is made specifically in the dimerisation reaction of cyclopentadiene"[2]. Therefore, the reaction is controlled kinetically rather than thermodynamically and the reaction intermediate in the formation of the endo product is at a lower energy than that for the exo formation. This can be explained by molecular orbital theory: The p orbitals have a more favourable overlap when approaching in an endo fashion (as shown) and so despite the increased strain (+1.8531 kcal/mol) induced, the less stable kinetically favoured endo product will form.
The dimerisation of cyclopentadiene is often reversible under reaction conditions, because of this the tendency of the less stable endo product is to revert back into the starting reactants or into the less sterically strained exo isomer.
Hydrogenation
|
Relative Energy Contributions (%) |
3 |
4 |
|
Stretching |
3.43 |
3.86 |
|
Bending |
52.50 |
44.56 |
|
Stretch-Bend |
-2.12 |
-1.94 |
|
Torsion |
34.08 |
42.31 |
|
Non-1,4 VDW |
-4.25 |
-4.46 |
|
1,4 VDW |
16.00 |
15.19 |
|
Dipole/Dipole |
0.45 |
0.48 |
|
Total |
100 |
100 |
Molecule 4 is made predominantly in the hydrogenation of cyclopentadiene dimer due to the increased bending (strain) induced in the formation of product 3. 44.56% of molecule 4's total energy is due to bending compared to 52.50% of molecule 3's. The hydrogenation reaction is therefore controlled thermodynamically with the most stable (lowest energy) product predominating.
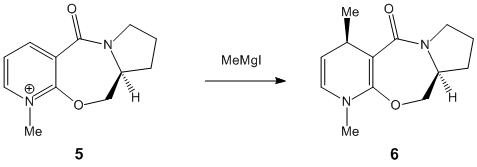
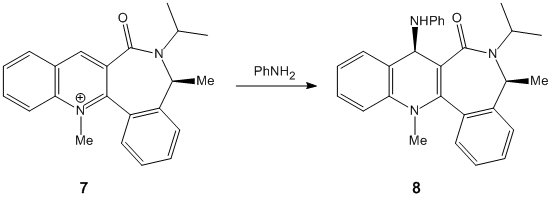
Stereochemistry of Nucleophillic Additions to a Pyridinium Ring
The aim of the following exercise is to use a molecular mechanics approach in order to justify the stereochemistry (shown below) in the products of two nucleophillic additions to two different pyridinium reactants.
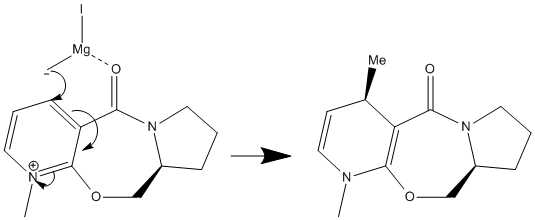
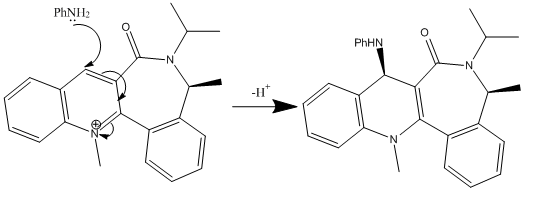
MM2 CALCULATIONS
|
Energy Contributions (kcal/mol) |
5
|
6
|
7
|
8
|
||||||||||||
|
Stretching |
0.8948 |
1.0047 |
1.5701 |
2.3597 |
||||||||||||
|
Bending |
6.3329 |
9.0993 |
6.7499 |
14.2319 |
||||||||||||
|
Stretch-Bend |
0.1159 |
0.2127 |
0.3371 |
0.6409 |
||||||||||||
|
Torsion |
9.8724 |
11.4384 |
-6.1357 |
0.1211 |
||||||||||||
|
Non-1,4 VDW |
-2.6366 |
-2.4613 |
-2.4273 |
-6.1747 |
||||||||||||
|
1,4 VDW |
11.5525 |
12.9424 |
17.6699 |
23.6768 |
||||||||||||
|
Charge/Dipole |
3.2500 |
- |
2.4124 |
- |
||||||||||||
|
Dipole/Dipole |
-3.7748 |
-3.8912 |
-4.8130 |
-4.4194 |
||||||||||||
|
Total Energy |
25.6078 |
28.3451 |
15.3633 |
30.4363 |
Orientation of Carbonyl Groups
|
Molecule |
Dihedral Angle(°) |
|
42.5 |
|
|
-40.0 |
Reactant 5: Carbonyl group is at an angle of 42.5° to the aromatic ring, anti to the hydrogen atom at the chiral centre. The methyl group in the product has the stereochemistry shown due to "chelation between the magnesium of the grignard reagent and the carbonyl oxygen"[5] forcing it outwards from the plane of the pyridine ring. Magnesium enolate is generated as the methyl carbanion is delivered to the pyridine C4 position. The product is higher in energy due to the loss of aromaticity in the pyridine ring.
Reactant 7: Carbonyl group is at an angle of -42.0° to the aromatic ring. The negative angle signifies a group pointing below the plane of the aromatic ring. The PhNH2 group attacking reactant 7 must approach from above the plane of the pyridine ring to prevent an electronic repulsion between the phenyl group and the downwards pointing carbonyl group. Again a loss of aromaticity in the product increases its total energy.
These simple models could be improved by including data about the energy of the transition states and intermediates. With this information a more complete argument could be made as to how the reactants come together and react in order to form the products with the stereochemistry shown above.
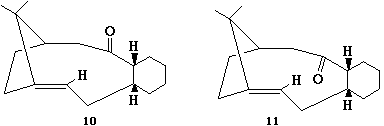
Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol
"A key intermediate in the synthesis of taxol is initially synthesised with the carbonyl group pointing either up or down. However, on standing the compound isomerises to the alternative carbonyl isomer (a process called atropisomerism)"[6]. The aim of this exercise is to determine which of the two isomers is the most stable and also to rationalise the abnormally slow reactivity of the alkene.
MM2 CALCULATIONS
|
Energy Contributions (kcal/mol) |
10
|
11
|
||||||
|
Stretching |
2.9872 |
2.5224 |
||||||
|
Bending |
18.7744 |
10.7327 |
||||||
|
Stretch-Bend |
0.5770 |
0.3278 |
||||||
|
Torsion |
23.8860 |
19.6680 |
||||||
|
Non-1,4 VDW |
-0.4033 |
-1.3499 |
||||||
|
1,4 VDW |
15.2084 |
12.5654 |
||||||
|
Dipole/Dipole |
0.1896 |
-0.1795 |
||||||
|
Total Energy |
61.2193 |
44.2868 |
From the MM2 calculations above it can be seen that molecule 11 is the most stable of the two. Molecule 11 has the carbonyl group pointing downwards. The cyclohexyl group in molecule 11 adopts a chair conformation with all the hydrogen atoms staggered and at the furthest possible distance apart from each other, lowering the energy. In molecule 10 however the cyclohexyl group is in a twist boat conformation. The twist boat conformation is higher in energy than the chair conformation due to a destabilising 1,4 VDW interaction between hydrogen atoms (flagpole interaction) and also due to eclipsed carbon bonds which raise the torsional strain. Both molecules are sterically hindered around the alkene which prevents electrophiles getting close enough in order for a reaction to take place.
How one might induce room temperature hydrolysis of a peptide
At room temperature and neutral pH "the halflife of a peptide bond is about 500 years".However, by careful conformational design organic chemists can achieve "esterification of a peptide like molecule in 20 minutes"[8]. The aim of the following exercise is to calculate the energies of two isomers of each of the reactants below and using these pairs of energies rationalise the kinetic behaviour.
1
 [9]
[9]
2
MM2 CALCULATIONS
|
Energy Contributions (kcal/mol) |
1
|
1
|
2
|
2
|
||||||||||||
|
Stretching |
1.6747 |
1.6460 |
1.5726 |
1.8739 |
||||||||||||
|
Bending |
8.4939 |
3.7322 |
5.7344 |
4.5621 |
||||||||||||
|
Stretch-Bend |
0.6419 |
0.5324 |
0.5701 |
0.5780 |
||||||||||||
|
Torsion |
11.7799 |
8.7992 |
10.0570 |
7.7318 |
||||||||||||
|
Non-1,4 VDW |
-6.9350 |
-4.2738 |
-5.3796 |
-4.2658 |
||||||||||||
|
1,4 VDW |
10.3007 |
10.0170 |
9.4262 |
9.9104 |
||||||||||||
|
Dipole/Dipole |
-3.6743 |
-3.5014 |
-4.5035 |
-3.3629 |
||||||||||||
|
Total |
22.2817 |
16.9517 |
17.4773 |
17.0274 |
In both of the starting reactants it can be seen that the most thermodynamically stable isomer is the one with the ethylamido group in the equatorial position, this is because the destabilising 1,3 diaxial interaction is minimised. For reactant 1 the hydrolysis reaction proceeds 40 times faster than reactant 2. This is due to the fact that for reactant 1 the isomer which has the carbonyl and OH group in close enough proximity to react is the thermodynamically more stable/more prevalent isomer. However, in reactant 2 the isomer which has the carbonyl and OH group close enough to react is the thermodynamically less stable/less prevalent isomer. Therefore, for reaction 2 to proceed the more thermodynamically stable isomer will first need to be converted into the less stable form at an energetic cost. Due to this energy barrier between the two isomeric forms the half life for the reaction is alot greater than would otherwise be expected.
Modelling using Semi-empirical Molecular Orbital Theory
Regioselective Addition of Dichlorocarbene
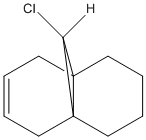
The diene shown reacts well with electrophillic reagents such as dichlorocarbene and peracids, it has been shown that these reagents react regiospecifically on the double bond endo to the chlorine substituent[10]. In the following exercise a PM3 valence-electron self-consistent-field MO method will be used in order to obtain a graphical representation of the frontier molecular orbitals. Once obtained, these MO's can be used to justify the regiospecificity of the diene.
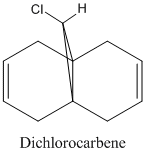
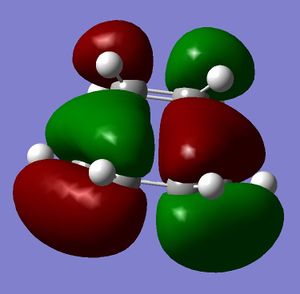
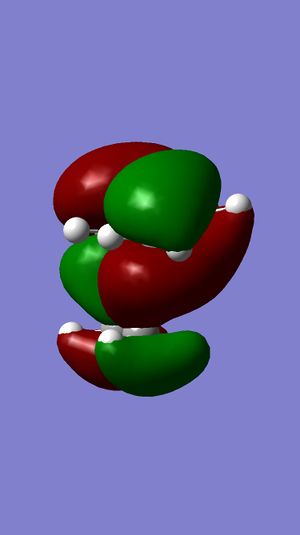
The regioselectivity of the diene can be explained by viewing the LUMO+2 and HOMO-1 molecular orbitals. The LUMO+2 MO shows a Cl-C σ* orbital which has a stabilising antiperiplanar interaction with the exo π orbital (shown in HOMO-1). This orbital interaction draws electron density away from the π orbital making the double bond less nucleophillic and therefore less likely to attack an electrophillic reagent. The overlap of molecular orbitals distorts the exo double bond in the direction of the bridgehead carbon. This can be shown by the shortened distance between one of the exo carbons and the bridgehead carbon.
Table showing distances between an endo or exo carbon and the bridgehead carbon
|
|
Endo (Angstroms) |
Exo (Angstroms) |
|
Diene |
3.28 |
3.02 |
|
Monohydrogenated diene |
3.25 |
3.07 |
|
3.36 |
The endo carbons are 0.26 Angstroms further away than the exo carbons from the bridgehead carbon atom due to this delocalisation of electron density from the exo π orbital into the C-Cl σ* orbital.
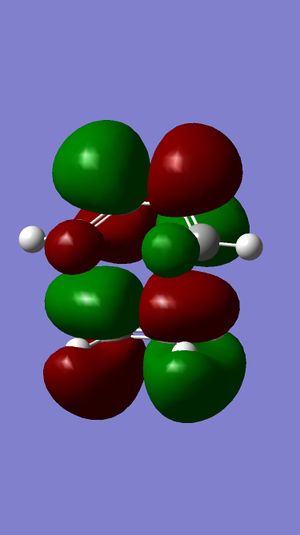
|
LUMO+2 |
LUMO+1 |
LUMO |
HOMO |
HOMO-1 |
 |
 |
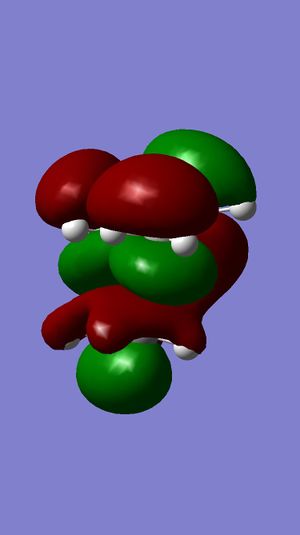 |
 |
 |
v=1/2π (k/μ)1/2
In the IR spectra for the diene, two peaks can be seen, one for each double bond. By using the above equation where k is the force constant (effectively the strength of the bond) the endo and exo stretching frequencies can be differentiated from each other. The exo bond is weaker (lower k value) due to electron density moving away from the exo π orbital and into the C-Cl σ* orbital. Due to the fact that the absorption frequency (v) is proportional to (k/μ)1/2 a lower frequency would then be expected. The monohydrated diene does not contain this orbital overlap so therefore the C-Cl stretching frequency would be expected to be higher.
IR absorption data (not all absorptions shown)
Structure based Mini project using DFT- based molecular orbitals
Assigning regioisomers in Click Chemistry
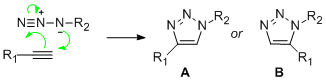
Click chemistry is a relatively new branch of organic synthetic chemistry discovered by K.B.Sharpless in 2001. Like the reactions that take in place in biological systems, click chemistry involves the joining of small simple molecules via heteroatom links in straighforward reactions to produce highly stereospecific complex molecules in high yields[11]. The Huisgen 1,3-dipolar cyclo addition is the most widely used click reaction and it has been said that this is one of the "rare organic reactions which approach perfection"[12]. This reaction involves the concerted addition of an alkyne (dipolarphile) and an azide (dipolar compound) (shown below). When this reaction is catalysed with a Cu(I) catalyst the 1,4 isomer (A) predominates in the product. However, when Ru(II) is used as the catalyst the 1,5 isomer (B) predominates in the product.
Spectroscopically the most effective way to tell these two regioisomers apart is the use of C13NMR since it gives information on the chemical environment of the nuclei and (if manipulated) coupling data. Infrared spectroscopy would not be a useful tool to differentiate between the two isomers because they both contain the same functionality.
In order to produce an NMR of the two isomers the geometry firstly had to be optimised. This was done initially on Chem Bio 3D as an MM2 calculation. Several different comformations were modelled in order to obtain the lowest energy conformation. This molecule was then optimised further by uploading a .gjf gaussian input file of the molecule onto an overnight gaussian SCAN system. A 6-31G(d,p) basis set was used for optimisation. Once the calculation had been completed the output file was downloaded, saved, then reuploaded as a .gjf for an NMR calculation, the results of which are below.
DFT optimisation
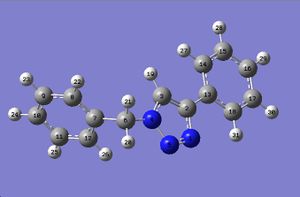
A
Pentahelicene |
|
Literature Compound (ppm) [14] |
Isomer A (ppm) |
|
54.0 |
57.90 (6) |
|
119.5 |
120.72 (3) |
|
125.5 |
124.74 (14), 125.29 (18) |
|
127.9 |
127.61 (12), 127.78 (16), 127.88 (8) |
|
128.56 |
128.26 (9), 128.33 (10) |
|
128.64 |
128.45 (15), 128.65 (17) |
|
128.9 |
128.96 (11) |
|
130.4 |
130.83 (13) |
|
134.6 |
136.44 (7) |
|
148.0 |
148.43 (2) |
The accuracy in the NMR for isomer A is reasonable compared to the literature values. The only value which stands out as being not so accurate is the one for carbon six, which is approximately 4ppm above the lit. value.
DFT optimisation

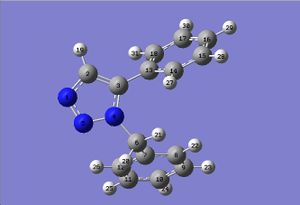
B
Pentahelicene |
|
Literature Compound (ppm) [15] |
B Isomer (ppm) |
|
51.85 |
55.85 (6) |
|
126.93 |
127.51 (9) |
|
127.22 |
127.90 (10) |
|
128.22 |
128.21 (14), 128.24 (12), 128.37 (8), 128.49 (11) |
|
128.92 |
128.61 (13), 128.72 (15), |
|
129.08 |
128.88 (17) |
|
129.64 |
129.36 (16) |
|
133.26 |
130.07 (18) |
|
133.34 |
133.61 (2) |
|
135.66 |
135.01 (7) |
|
138.26 |
139.93 (3) |
The accuracy in the NMR for isomer B is not as good as that for isomer A (compared to the literature values). Again carbon six is 4ppm off the lit value. Carbon six is a methylene carbon, the reason for the inaccuracy of this shift could be due to miscalculation, however the other chemical shifts are not far off what is reported in the literature so this is highly unlikely, another possibility is that the calculation is not taking into account a certain parameter, but then again any new parameter introduced into the calculation could possibly affect all of the other correct chemical shifts. Considering the whole calculation model is based on approximations the method is a very affective way of producing an NMR spectrum with a high amount of accuracy in a relatively short time period.
The noticeable difference between the two NMR spectrum is the absence of the peak at 148.43 ppm in the NMR for Isomer B. Carbon two is responsible for this peak. The peak carbon two actually generates in NMR B is 133.61 ppm. The difference in chemical shift between these two carbons is due to the phenyl ring which is attached to carbon two in isomer A. The phenyl group is part of an extended planar delocalised system which deshields carbon two, increasing its chemical shift. This delocalised system is still present in isomer B however, the phenyl ring is no longer in the same plane as the triazole, reducing the deshieling effect, decreasing the chemical shift. This particular chemical shift can be used to identify which of the regioisomers is present in the products.
References
- ↑ http://www.ch.ic.ac.uk/wiki/index.php/Mod:organic
- ↑ http://www.ch.ic.ac.uk/wiki/index.php/Mod:organic
- ↑ http://www.ch.ic.ac.uk/wiki/index.php/Mod:organic
- ↑ http://www.ch.ic.ac.uk/wiki/index.php/Mod:organic
- ↑ A. G. Shultz, L. Flood and J. P. Springer, J. Org. Chemistry, 1986, 51, 838. DOI:10.1021/jo00356a016
- ↑ http://www.ch.ic.ac.uk/wiki/index.php/Mod:organic
- ↑ http://www.ch.ic.ac.uk/wiki/index.php/Mod:organic
- ↑ https://www.ch.ic.ac.uk/wiki/index.php/Mod:organic
- ↑ https://www.ch.ic.ac.uk/wiki/index.php/Mod:organic
- ↑ B. Halton, R. Boese and H. S. Rzepa., J. Chem. Soc., Perkin Trans 2, 1992, 447. DOI:10.1039/P29920000447
- ↑ S. D. Gonzalez and S. P. Nolan, Angew.Chem.46/2008, 8881-8884 DOI:10.1002/anie.200803289
- ↑ J. F. Lutz, Angew. Chem. 7/2007, 991-995 DOI:10.1002/anie.200604050
- ↑ http://www.ch.ic.ac.uk/wiki/index.php/Mod:organic
- ↑ U. Sirion, Y. J. Bae, B. S. Lee and D. Y Chi, Synlett, 2008, 2326-2330DOI:10.1055/s-2008-1078245
- ↑ L. Zhang, X. Cheng, P.Xue, H. H. Y. Sun, I. D. Williams, K. B. Sharpless, V. V. Fokin and G. Jia, 2005, J. Am. Chem. Soc., 127(46), 15998-15999 DOI:10.1021/ja054114s