Rep:Mod:atbxz79364
The Pathway to Adamantane
In Module 1[2], we looked at the dimerisation of Cyclopentadiene, via a Diels Alder Cycloaddition to give endo-dicyclopentadiene only (at room temperature), which we rationalised as being due to a more favorable transition state for this diastereoisomer, due to secondary orbital overlap from the other alkene bond. We shall re-investigate this reaction here, using the methods we have learnt, and compare to the qualitative picture we formed earlier.
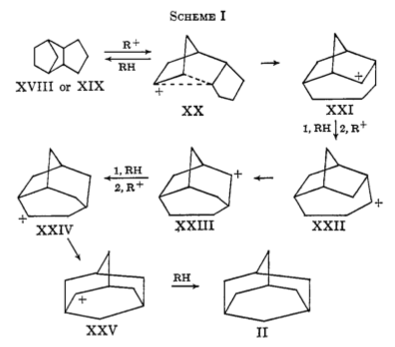
Also, when looking through literature whilst studying that reaction, I found a paper[1], presenting a synthesis of the diamonoid adamantane, by rearrangement of endo-tetrahydrodicyclopentadiene in the presence of AlCl3. AlCl3 acts as a carbocation generator, which is proposed to initiate the reaction. Then, through a series of intermediates, the endo-structure rearranges to give adamantane. The authors proposed a rearrangement pathway.
Whilst there is not sufficient time to fully investigate this proposed pathway, there is one key step, from intermediate XX to XXI, a 2,6-alkyl migration, which the authors describe as "(having) no direct precedent and is therefore subject to some suspicion. Analogous 2,6-hydride shifts are well documented however, and a 2,6-methyl migration has been observed in a carbenoid reaction. The postulated alkyl migration in the adamantane rearrangement therefore is not altogether unreasonable."[1] This step will be examined, by modeling the reactant and product and looking for the transition state and reaction path, to determine its feasibility.
The Dimerisation of Cyclopentadiene
Reactants and Products
First, as always, we need to optimise the reactants and products. Cyclopentadiene was created using a Gaussview fragment, and optimised initially to HF 3-21G, then using that result, to DFT B3LPY 6-31G*. Because the molecule is a diene, it is necessarily planar, so we don't have to search for other conformations.

The symmetry allowed products of this cycloaddition are endo- and exo-dicyclopentadiene, though as we have said, only the endo- forms at room temperature. These products were created using Gaussview, and various bicyclic fragments, and carbon tetrahedral fragments, adjusting bond lengths, types and angles accordingly. These guess structures were then optimised to HF 3-21G then DFT B3LYP 6-31G* theory. Again, these molecules are conformationally locked, so we are not concerned with other minima.
We find the exo-dimer to be 1.10 kcalmol-1 more stable than the endo-dimer. This agrees with our MM2 conclusion, which we argued was due to the steric bumping across the molecule in the endo-form, as it folded back on itself. In the exo form, the two rings are removed from each other, so this bumping is removed.
|
Reactant and Product Energies /DFT B3LYP 6-31G* |
||
|
Molecule |
Energy /Eh |
Energy /kcalmol-1 |
|
Cyclopentadiene |
-194.101058 |
-121800.3549 |
|
Endo-DCPD |
-388.2280216 |
-243616.9658 |
|
Exo-DCPD |
-388.2297763 |
-243618.0669 |
Transition States
Because the higher-energy endo form is the only product we can say that this is a kinetically driven process. This means that the transition state to the endo- form is of lower energy than that to the exo form. We use our newly learnt techniques to show this to to be the case.
To find the transition states, the QST2 method was used, starting from two cyclopentadiene molecules, separated in space, in the correct relative orientations to each other, and the corresponding product diastereoisomer, with the atomic labeling changed accordingly.
|
Dicyclopentadiene Cycloaddition QST2 |
||||||
|
Starting Point |
End Point |
Result |
||||
|
Exo-Isomer |
|
|||||
|
Endo-Isomer |
|
|||||
The interpolation between the two sets of atomic positions and then subsequent transition state optimisation carried out by QST2 resulted in the structures shown below. As was the case for the two previous Diels Alder reactions studied, we cleaned up the transition state in Gaussview, removing the false connections shown in the interface. The vibrational modes of the transition states were calculated, and in each isomer, one imaginary frequency was found. In the endo-isomer this had magnitude 652cm-1, and in the exo-isomer, had magnitude 719cm-1. Animating these modes once more shows us the displacement characteristic of the Diels Alder reaction.
|
Transition State Energies /DFT B3LYP 6-31G* |
||
|
Molecule |
Energy /Eh |
Energy /kcalmol-1 |
|
Endo-DCPD TS |
-388.1711242 |
-243581.2621 |
|
Exo-DCPD TS |
-388.1667293 |
-243578.5043 |
IRC: Reaction Pathway


From the results of the QST2 TS optimisation, an IRC calculation was set up on each of the isomeric transition states, specifying iteration in both directions. To HF 3-21G theory. The resulting energy profiles and geometries at key points are shown below.
As the reactants approach each other, their energy rises, as steric and electronic repulsions begin to increase. As the reactants begin to change their conformations to approach the transition state, the energy rises at a steeper rate. As the new σ bonds form, the energy quickly drops, and a negative reaction enthalpy results. Both pathways have late-transition states.
By plotting the absolute energy against the reaction coordinate for both pathways together, we see two things. One, that the exo product is slightly lower in energy than the endo product. Two, that the endo transition state is lower in energy than the exo transition state. This is in agreement with the qualitative picture we formed back in Module 1; that the endo kinetic product forms because its energy of activation is lower than the exo form, which we put down to favorable, transition state stabilising secondary orbital overlap, as we saw for the case of Maleic Anhydride above. There, a pi system from the carbonyl function stabilised the transition state. Here, is it the pi system from the other double bond of the cyclopentadene dienophile.
Finally, we report the activation and free energy changes for reaction, to DFT B3LYP 6-31g* theory. The Endo Pathway activation energy is calculated at 19.45 kcalmol-1, wheras the exo-pathway actvation energy is calculated at 22.21 kcalmol-1. The endo enthalpy change on reaction is -35.70 kcalmol-1, and that for the exo is -39.56 kcalmol-1.
A 2,6-Alkyl Shift?
Optimising XX: The endo-tetrahydrodicyclopentadiene cation


From the resulting geometry of the endo-dicyclopentadiene calculation, the two double bonds were redefined as single bonds and the lengths changed, and the valency of the carbon atoms changed accordingly. Then, one hydrogen atom was removed, and the charge of the system increased to +1 in the input file. Then, an optimisation was carried out to DFT B3LYP 6-31G* theory.
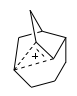
If we follow the optimisation procedure, we find that, initially, the apex with only one H atoms initially becomes planar, as expected, since this, classically, the structure of a carbocation, but then we find the system is able to further lower its energy, by delocalising that charge over three carbon centres, by dissociation of a single bond. This is an example then, of a non-classical cation. If we look at the vibrational modes, we find no imaginary frequencies, confirming that this is a minimum and not a transition state.
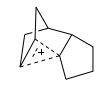
The jmol above shows the region of delocalisation of the charge, by looking at the valence and structure of the corresponding C atoms. Hence, the graphic above, from the paper, would be better represented as that shown to the right:
|
Intermediate XX Structure |
|||
|
Optimising XXI

To find the minimum in the XXI intermediate, first, a neutral molecule was created and optimised to HF 3-21G, the using the result of this, a hydrogen was abstracted from the correct position as before, to give a structure looking much like that to the left, and reoptimised to DFT B3LYP 6-31G*. A plot of the optimisation path is shown below.

Again, initially, the molecule becomes planar at the low-valency apex, before again delocalising to give a non-classical cation, spread over three vertices. The representation given by the authors is poor, as it shows localised character of the cation. This as we have found is not a stable conformer, but lowers its energy further by spreading the charge. The structure is better represented as that shown:
|
Intermediate XXI Structure |
|||
|
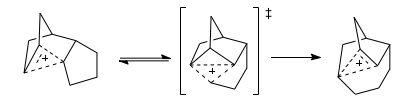
Finding the Transition state
With the complex molecular geometry in the product and reactant, forming a guess transition state would be difficult. However, this looks like a good job for QST2: most of the molecule is fixed, and in the reactant and product the same non-classical cation is seen, on opposite faces of the molecule. Hence, an interpolation between these is a good start to look for our transition state. This was carried out, numbering the reactant and product accordingly, to DFT B3LYP 3-21G Theory.
The resulting geometry predicted for the transition state shows the terminal C atoms of the alkyl chain to migrate mid way between the vertex it left and that which it is going to. Looking at the vibrationa, we find one imaginary mode at a magnitude of 387cm-1, which if we animate shows the displacement back and forth of the terminal C atoms of the alkyl chain. This suggests we have found to correct transition state for this alkyl migration.
We can graphically represent this transition state as having its charge again delocalised over three centres, with the alkyl termini mid point passing over the front face of the system. To confirm that this transition state is that for this rearrangement process, we will conduct an IRC calculation.
|
Adamantane Rearrangment XX to XXI TS |
|||
|
Following the reaction Path

An IRC calculation was carried out on the transition state geometry, specifying iteration in both directions. The resulting energy profile was plotted, show below with geometries at key points. To HF 3-21G theory.
Click here to see the IRC path...
We see the energy quickly rise as the geometry changes to allow the alkyl group to migrate. Previously, we tended to see a slow energy rise initially, when we considered bimolecular reactions, as the reactants moved toward each other. However, this is unimolecuular, so the need to adjust the structure means a steep energy rise. The energy reaches a maximum as the alkyl group migrates mid way between the system. This is because the delocalised charged three centre system has been distorted heavily and 'stretched' if you like. Then, as the non-classical cation reforms the other side, the energy drops.
The calculated activation energy on going from intermediate XX to TS is 26.44 kcalmol-1. The reaction from XX to XXI is very slightly endothermic with a reaction enthalpy of +0.77 kcalmol-1.
If this process possible? The authors proposed the pathway based upon analysis of the strain of the system - the highly strained endo-tetrahydrodicyclopentadiene rearranging to release strain. However, as we can see from the energies, there is no relief of strain, and the associated gain in stability, because the energy of the starting material and product are very similar, in fact the energy of the product as defined here is slightly higher in energy! This is because the two systems are both equally strained- by delocalising the charge, the systems are able to reduce some strain already, so the molecule is not confined to the bicyclic geometry, which the authors predicted above. So thermodynamically, there is no desire for this process to occur. But the energy difference is minimal between the two carbocations, so we would expect an equlibrium to exist between the two. The transition state energy is comparable to the Diels Alder reactions we previoously considered, so we expect it to be passable. Hence, we can say this process may be occuring, to set up an equlibrium between the two, but there is no great preference for either system. If the next reaction from XXI is fast, this could be removed from equlibrium and we would slowly see XX disappear, as XXI is converted. Hence, this step could be involved.
|
Rearrangement Species Energies /DFT B3LYP 6-31G* |
||
|
Intermediate: |
Energy /Eh |
Energy /kcalmol-1 |
|
Intermediate XX |
-389.7989889 |
-244602.7635 |
|
Transition State |
-389.7568615 |
-244576.3281 |
|
Intermediate XXI |
-389.8002186 |
-244603.5352 |
- ↑ 1.0 1.1 1.2 R.C.Fort, J.R.Schleyer, P.VR.Schleyer, Chem. Rev. 1964, DOI: 10.1021/cr60229a004
- ↑ Philip Murray, Organic Compuational Lab, https://wiki.ch.ic.ac.uk/wiki/index.php?title=Mod:xxyte35130, 2010
General references made throughout to:
M.Bearpark, https://wiki.ch.ic.ac.uk/wiki/index.php?title=Mod:phys3, 2008
J.B.Foresman and A.Frisch, Exploring Chemistry with Electronic Structure Methods, 1996