Rep:Mod:asdfgh1234
NH3 molecule
N-H bond distance = 1.01798
H-N-H bond angle = 37.129
Key information from optimised job
Molecule name = NH3
Calculation method = RB3LYP
Basis set = 6-31G(d,p)
Final energy E(RB3LYP) in atomic units = -56.55776873
Point group of molecule = C3V
Item table
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000072 0.001800 YES
RMS Displacement 0.000035 0.001200 YES
NH3 Molecule |
The optimisation file is linked to here
Vibrations and charges
-6 modes would be expected from the 3N-6 rule
-Modes 2 + 3 and 5 + 6 are degenerate (ie have the same energy)
-Vibration modes 1, 2 + 3 are bending. Whereas, 4, 5 + 6 are stretching
-Mode 4 is highly symmetric
-Mode 1 is known as the "umbrella" mode
-You would expect to see 6 bands in an experimental spectrum of gaseous ammonia
Charge distribution
We would expect hydrogen to be positive and, as a consequence, the nitrogen to be negative
H = 0.375
N = -1.125
N2 Molecule
N-N bond distance = 1.09200
Key information from optimised job
Molecule name = Nitrogen gas
Calculation method = RB3LYP
Basis set = 6-31G(d,p)
Final energy E(RB3LYP) in atomic units = -109.52359111
Point group of molecule = D*H
Item table
Item Value Threshold Converged?
Maximum Force 0.000001 0.000450 YES
RMS Force 0.000001 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000000 0.001200 YES
There was just one mode of vibration identified; frequency = 2457.33, infrared = 0
H2 Molecule
H-H bond distance = 0.60000
Key information from optimised job
Molecule name = Hydrogen gas
Calculation method = RB3LYP
Basis set = 6-31G(d,p)
Final energy E(RB3LYP) in atomic units = -1.15928020
Point group of molecule = D*H
Item table
Item Value Threshold Converged?
Maximum Force 0.000000 0.000450 YES
RMS Force 0.000000 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000001 0.001200 YES
There was just one mode of vibration identified; frequency = 4465.68, infrared = 0
Reaction energies
N2 + 3H2 --> 2NH3
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -0.11410576 a.u
-0.11410576*2625.5 = -299.58 kJ/mol
Choice of Small Molecule
Molecule = CH4
C-H Bond distance = 1.07000
Bond angle = 109.471
Key information from optimised job
Molecule name = Methane
Calculation method = RB3LYP
Basis set = 6-31G(d,p)
Final energy E(RB3LYP) in atomic units = -40.52275298
Point group of molecule = T
Item table
Item Value Threshold Converged? Maximum Force 0.000063 0.000450 YES RMS Force 0.000034 0.000300 YES Maximum Displacement 0.000179 0.001800 YES RMS Displacement 0.000095 0.001200 YES
CH4 Molecule |
The optimisation file is linked to here
Vibrations and charges
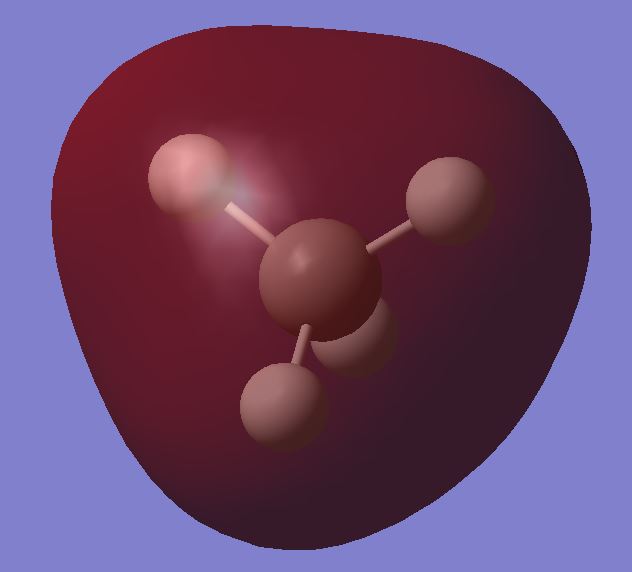
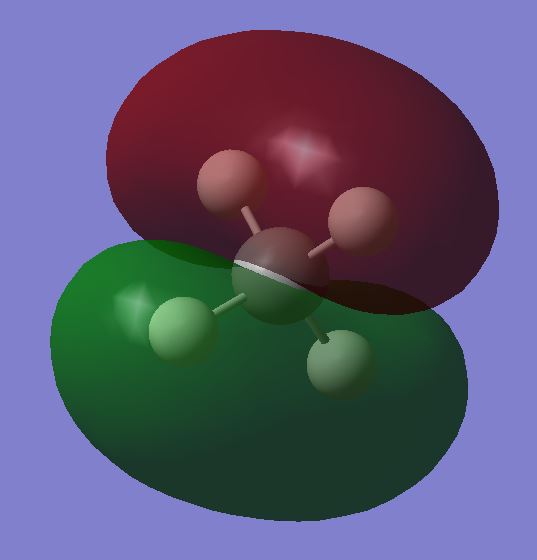
Molecular Orbitals
Worth revisiting this when I am with the group in Chemistry
This MO is deep in energy. No overlap, held tightly to nuclei and not involved in chemical bonding
Combination of valence orbitals bonding and antibonding. Strong overlap, likely to be involved in chemical bonding
Orbitals lie along the bond. Sit side by side, etc.
Charge distribution
H = 0.233
C = -0.930