Rep:Mod:apb315
Appearance
Compound Analysis
NH3
- Name: Ammonia
- Calculation Method: RB3LYP
- Basis Set: 6-31G(d,p)
- Final Energy E(RB3LYP)= -56.55776873 a.u.
- Point Group: C3V
- Bond Length= 1.01798 Å
- H-N-H Bond Angle= 105.741°
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES
Optimised model of NH3 |
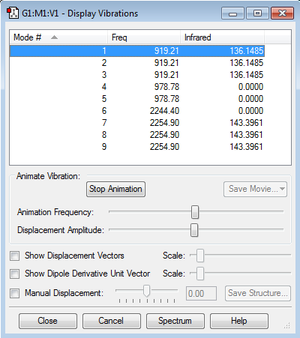
Display Vibrations
None of the frequencies are negative so we have a minimum.
Questions
- Using the 3N-6 rule you would expect 6 vibrational modes for NH3.
- Modes 2 and 3 are degenerate and modes 5 and 6 are degenerate.
- Modes 1, 2 and 3 are "bending" vibrations. Modes 4,5 and 6 are "stretching" vibrations.
- Mode 4 is highly symmetric.
- Mode 1 is known as the "umbrella" vibrational mode.
- You would expect to see 4 bands in an experimental spectrum of gaseous ammonia.
Charge
- The charge on the N atom is -1.125.
- The charge on the H atoms is 0.375
- You would expect the N to be negatively charged and the H atoms to be positively charged because N is more electronegative than H.
N2
- Name: Nitrogen gas
- Calculation Method:RB3LYP
- Basis Set: 6-31G(d,p)
- Final Energy E(RB3LYP)= -109.52412868 a.u.
- Point Group: D∞h
- Bond Length= 1.10550 Å
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
Optimised model of N2 |
Display Vibrations
None of the frequencies are negative so we have a minimum.
Charge
The charge on both N atoms is 0.000.
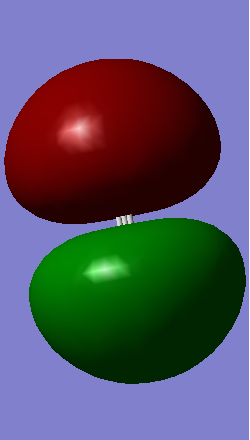
Molecular Orbitals
H2
- Name: Hydrogen Gas
- Calculation Method:RB3LYP
- Basis Set: 6-31G(d,p)
- Final Energy E(RB3LYP)= -1.17853936 a.u.
- Point Group: D∞h
- Bond Length= 0.74279 Å
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES
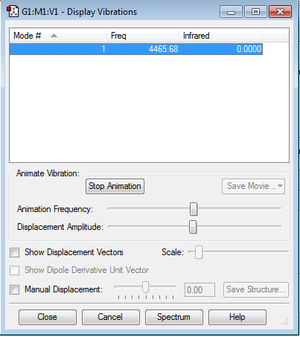
Optimised model of H2 |
Display Vibrations
None of the frequencies are negative so we have a minimum.
Charge
The charge on both H atoms is 0.000.
Reaction Energies
- E(NH3)= -56.55776873 a.u.
- 2*E(NH3)= -113.11553746 a.u.
- E(N2)= -109.52412868 a.u.
- E(H2)= -1.17853936 a.u.
- 3*E(H2)= -3.53561808 a.u.
- ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -0.0557907 a.u.
- ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -146.48 kjmol-1
- The NH3 product is preferred over the reactants. The product is more stable than the reactants.
SiH4
- Name: Silane
- Calculation Method: RB3LYP
- Basis Set: 6-31G(d,p)
- Final Energy E(RB3LYP)= -291.88802760 a.u.
- Point Group: Td
- Bond Length= 1.48485 Å
- H-Si-H Bond Angle= 109.475°
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
Optimised model of SiH4 |
Display Vibrations
- None of the frequencies are negative so we have a minimum.
- Modes 1, 2 and 3 are degenerate. Modes 4 and 5 are degenerate. Modes 7,8 and 9 are also degenerate.
- You would expect to see 3 bands in an experimental spectrum of SiH4. This is because there are only three unique infrared values in the table.
Charge
- The charge on the Si atom is 0.629.
- The charge on the H atoms is -0.157.
- You would expect the H atoms to be negatively charged and the Si atom to be positively charged because H is more electronegative than Si.
Molecular Orbitals
Mouse over for figure numbers.
- Figure 1 has an energy of -5.28056 a.u.
- Figure 2 has an energy of -3.63858 a.u.
- Figure 3 has an energy of -0.35184 a.u.
- Figure 4 has an energy of 0.05053 a.u.
- Figure 5 has an energy of 0.22050 a.u.