Rep:Mod:ap1707
Anshumalita Patel
MODULE 1
Molecular Mechanics Modelling
In the following, a computer is used to model various aspects of the structures and reactivity of certain organic molecules. This is helpful in justifying experimental findings which are sometimes unexpected. This type of modelling can also be used to inspect what useful changes can be made to the molecule in terms of reactivity and stability in other reactions.
1.1 Isomerisation and Hydrogenation of Diyclopentadiene
Dicyclopentadiene is formed via a Diels-Alder cycloaddition and this can yield either the exo- (1) or endo- (2) product depending on which face the dienophile attacks but at room temperature it dimerises to give the endo isomer exclusively:
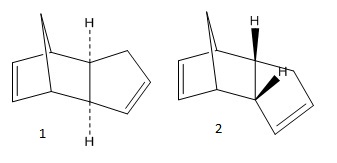
To rationalise this exclusivity an MM2 calculation was performed on ChemBio3D and the total energies for both dimers were found. The energy for the exo- product is 31.88 kcal/mol which is lower than the 34.01 kcal/mol found for the endo- product. This is due to the steric hindrance in the endo- form of the two rings pointing inwards towards one another making it slightly less stable. It is then explicit that the dominating endo- form must be the kinetic product. It is formed faster as it goes via a lower energy transition state and it predominates because the Diels-Alder reaction is irreversible.
On hydrogenating the endo-isomer, the regioisomers 3 and 4 can be formed. To predict the ease of hydrogenation the contributions from the various energy terms were calculated for both the dihydro derivatives.[1] :
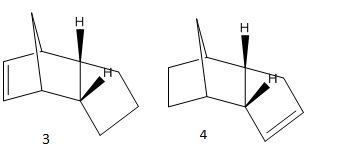

The dihydro derivative 4 has a lower bonding, stretching, torsional and total energy than derivative 3. This is expected as the norbornene C=C bond experiences more strain than the double bond in the cyclopentene ring due to the bridging methylene group (manifested in a greater deviation in the bond angles from the ideal 120° for an sp2 carbon)[2]. Thus there is a greater relief of strain when this bond is reduced as opposed to that in molecule 3 and so it would be expected that this will be the favoured hydrogenation.
1.2 Stereochemistry of Nucleophilic additions to a pyridinium ring (NAD+ analogue)
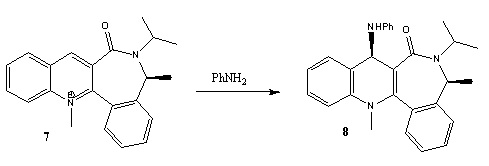
The following two reactions are analysed to investigate the observed stereochemistry of the products.The first reaction involves a derivative of prolinol (5) which reacts with MeMgI to alkylate the pyridine ring and give the product (6). In the second reaction, molecule (7) is reacted with aniline to give (8).
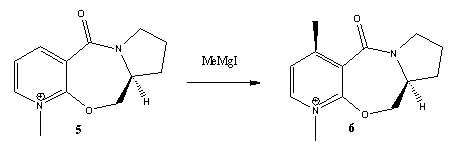
Reaction 1
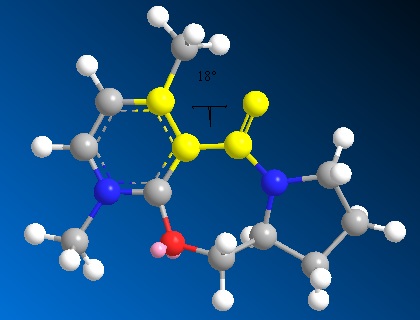
The MM2 force field in ChemBio3D is used to minimise the energy of both the reactants (5) and (7).The atoms were then manually moved to find the minimum energy possible.Movement of the carbonyl and the seven-membered ring was fairly constrained and so the main changes which affected the energy were those on the five-membered ring. The minimum energy found was 43.18 kcal/mol with a dihedral angle of about 25°. Other conformers of higher energies were also found and one is shown below with a energy of 47.86 kcal/mol and dihedral angle of 18°. The absolute stereochemistry observed can be understood by looking at the mechanism[3]:

The Mg in the Grignard reagent coordinates to one of the oxygen lone pairs thus leading the methyl group to attack over the top face of the molecule and giving the resulting stereochemistry seen in (6). Magnesium cannot be included in the MM2 calculation because it runs on set parameters that do not include magnesium.
Reaction 2
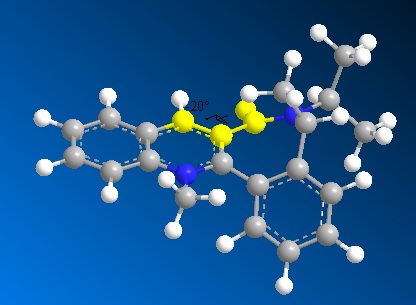
Molecule (7) was manipulated in a similar way, mainly by moving the carbonyl group up and down, to find the lowest energy conformer. Again the size of the large ring system did not allow for significant changes in geometry. The lowest energy found was 62.07 kcal/mol with a dihedral angle of -20°. Further manipulations only yielded very small changes in energy e.g 62.60 kcal/mol.
Unlike the Grignard reagent, the aniline nucleophile cannot coordinate to the carbonyl oxygen so the stereocontrol for this nucleophilic addition arises from the steric hindrance of this large group as can be seen in the mechanism below:
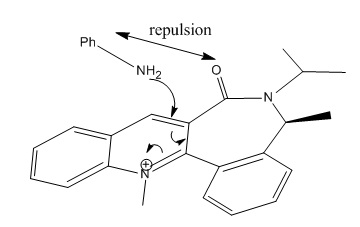
The aniline adds to the top face of the ring and directs itself away from it-as can be seen in (8)- so as to avoid this hindrance.
An improvement to this modelling would be to avoid the inaccuracies stemming from the simplistic MM2 force field and its use of set parameters and using a more advanced type. Something which also took into account the interactions of electrons in the different orbitals would be far more accurate. Results could also be improved if the Mg atom in the first reaction could have been included by adding more parameters to the force field.
1.3 The stereochemistry and reactivity of a Taxol synthesis intermediate
It has been shown by Paquette[4] that an intermediate (9 or 10 shown below) in the synthesis of Taxol is formed with the carbonyl pointing either up or down. MM2 and then MMFF94 force fields will be used to find out which atropisomer is the most stable.
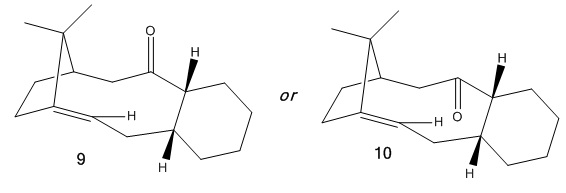
Firstly Intermediate 9 was drawn in ChemBio3D and an MM2 minimisation was performed, yielding an energy of 54.37 kcal/mol. It was seen that in the optimised structure the six-membered ring was in the higher energy boat conformation and so to reduce the energy further it was changed to the chair conformation. Along with other minor edits of the atom positions the energy was lowered to 48.98 kcal/mol. A similar process was carried out with Intermediate 10 which was initially found to have an energy of 51.27 kcal/mol but was then reduced by again switching to the chair conformation to give an energy of 45.52 kcal/mol. From looking at the energy contributions below, it seems that the main cause for the difference in energy between the two intermediates is the decrease in bending when the carbonyl is moved to a downward position.
These final structures were then minimised using an alternative force field (MMFF94)- which gave energies of 70.64 kcal/mol for Intermediate 9 and 61.39 kcal/mol for Intermediate 10, correlating well with the MM2 calculations and showing that 10 is the more stable isomer.
Intermediate 9
9 |
Intermediate 10
Intermediate 10 |
It has also been noted that the alkene in these isomers is exceptionally unreactive. This is surprising as it would be expected to be highly unstable due to the strain caused by its position at the bridgehead. However this can be rationalised by the fact that the ring is large (10-membered) and so allows for the transannular bonding without too much difficulty. In fact what is actually occurring here can be explained by an effect known as hyperstability[5]. It is found that in rings of this size it is actually preferable to have sp2 carbons at the bridgehead rather than sp3 carbons as these would cause more strain. Therefore it is unfavourable to react the alkene and thereby remove the two sp2 centres as this would lead to greater transannular strain. Thus explaining why this reaction is so slow.
1.3 Regioselective Addition of Dichlorocarbene
We are now going to take into account electronic aspects of reactivity to analyse the addition of dichlorocarbene to the molecule 12 shown below:

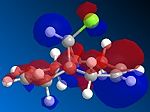
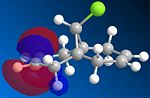
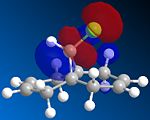
The dichlorocarbene adds endo orsyn to the chloro substituent and experimentally the second molecule with adducts on both alkenes is not detected. This regiospecificity is controlled by the orbitals involved so we need to use a program that can predict the geometry, calculate the orbital energies and display them graphically. Therefore the molecule was first drawn in ChemBio3D and the energy minimized using MM2 (to yield an energy of 17.8971 kcal/mol) which optimized the geometry and then the MOPAC/PM6 method was used to give approximate representations of the molecular orbitals important in the reaction of the alkene with an electrophile. These orbitals being the HOMO, LUMO and the orbitals close to them (HOMO-1 and LUMO+1)are important because the electrons from the HOMO will be donating its electrons to the dichlorocarbene.
| HOMO-1 | HOMO | LUMO | LUMO+1 | LUMO+2 |
|---|---|---|---|---|
 |
 |
 |
 |
 |
We can now understand why the electrophilic dichlorocarbene attacks the syn alkene as by looking at the HOMO orbital we can see that there is a lot more electron density on this bond as opposed to the anti alkene. By looking at the representation of the HOMO-1 orbital we can also see that it would indeed be possible to react anti to the chlorine but only after the syn-alkene is reacted. So it can be concluded that the syn-alkene is the more nucleophilic and is preferred for attack.
Next the vibrations were looked at for molecule 12 and its hydrogenated anti-alkene.

For Molecule 12 the C=C stretch for the anti alkene was 1740cm-1 and for the syn it was 1761cm-1 which essentially shows that the anti alkene has a lower bond strength and is therefore more reactive than the syn. This, however, is not what is seen in the above reaction. The result may be due to the fact that sigma orbitals are taken into account for the frequency calculations as well as the bonding pi orbitals which were looked at in the addition reaction. Therefore though the bond is weaker, the syn pi orbitals must be better at donating electrons than the anti so the bond energy does not really determine their reactivity. The stretch for the syn alkene in the hydrogenated molecule is the same (1761cm-1). For the C-Cl stretches, molecule 12 had a frequency of 772cm-1 but when the anti alkene was removed, the frequency increased to 776cm-1. This could be due to loss of stabilisation/orbital overlap of the C-Cl orbitals and the alkene.
1.4 Mini Project- Stereoselective Dissolving Metal Reductions
The reduction of the cyclic ketone (5) below gives only one stereoisomer of the resulting alcohol (6). The conditions for the reaction were found in the report by Castellanos et al.[6] as was the following reaction scheme with the numbered ketone:

It is quite easy to see if this reaction has occurred as if we look at IR spectra of the reactant and the product we should see that the carbonyl peak for the reactant will have disappeared in the product spectrum and so instead of the peak at 1715cm-1 typical for 6-membered cyclic ketones we will see a peak between 3200cm-1 and 3500cm-1 for the alcohol stretch. To be able to tell which isomer was formed we would have to look at the differences between the two. In 6 the hydroxyl on carbon 3 is equatorial whereas in 7 it is axial (assuming that the conformation of the decalin locked by its three alkyl substituents). Therefore the only thing that will have changed is the interaction of the hydrogen on this hydroxyl atom with the rest of the molecule. In isomer 7 the hydrogen is gauche to the two hydrogens on carbon 2 and the hydrogen on carbon 3 however in isomer 6 the hydrogen is anti-periplanar to one of the hydrogens on carbon 2 and gauche to the other, as well as being anti-periplanar to a hydrogen on carbon 4. So now we know the differences in the interactions of both the hydrogens we can tell which isomer is formed by looking at the H-coupling on the spectrum.Because the two hydrogens that are interacting are three atoms away from one another we will specifically be looking at the 3J coupling. This type of coupling is dependent on the angle between the two atoms so for a anti-periplanar relationship the angle will be 180° and the resulting coupling will be 11Hz and for the gauche, the angle is 60° so the resulting coupling will be 3Hz.
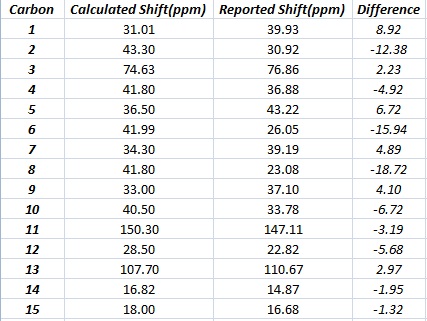
The calculated values correlate well with the literature values for the most part although the values for carbons 2,6 and 8 had a slightly larger difference than the rest.
References
- ↑ D.Skala and J.Hanika, Petroleum and Coal, Vol 45, 105-108, 2003
- ↑ DOI:10.1016/S0040-4039(00)87361-X
- ↑ Schultz et al.J. Org. Chem., 1986, 51 (6), pp 838–841
- ↑ S. W. Elmore and L. Paquette, Tetrahedron Letters, 1991, 319 : DOI:10.1016/S0040-4039(00)92617-0
- ↑ Kukuk et al. Angew Chem. Suppl. 1982. 696-701
- ↑ Castellanos et al., Tetrahedron Letters, 2006 : DOI:10.1016/j.tet.2006.12.019