Rep:Mod:annyding
Part One
The Hydrogenation of Cyclopentadiene Dimer
Cyclodimerisation and Hydrogenation
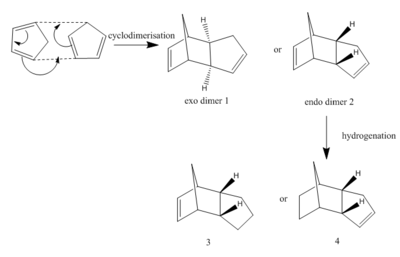
At room temperature, cyclodimerisation occurs between two cyclopentadienes to produce either exo dimer 1 or endo dimer 2. Cyclopentadiene dimerises to produce only endo dimer 2. The objective is to figure out whether this reaction is controlled kinetically or thermodynamically by calculation of the geometries and energies of the two dimers. The structures of dimer 1 and 2 have drawn by Chemdraw 3D and their geometries were optimized by Avogadro under MMFF94(s) force field option; as well as the bond stretching, angle bending, torsional, out-of-plane, van der waals and electrostatic energies. The results are shown below in the table 1 and further analyzed later.
The endo dimer 2 can undergo a further hydrogenation leading to two new molecules 3 or 4 (after a long time). Using the same approach, compounds 3 and 4 have been optimized, and the results are shown in table 1 as well.
The rotatable models of exo dimer 1, endo dimer 2, molecule 3 and molecule 4 are displayed below in this order.
Pentahelicene |
Pentahelicene |
Pentahelicene |
Pentahelicene |
| Molecules | 1(kcal/mol) | 2(kcal/mol) | 3(kcal/mol) | 4(kcal/mol) |
|---|---|---|---|---|
| Total Bond Stretching Energy | 3.54699 | 3.4654 | 3.31534 | 3.04019 |
| Total Angle Bending Energy | 30.80127 | 33.23727 | 32.09876 | 26.97620 |
| Total Stretch bending Energy | -2.03866 | -2.07488 | -2.08019 | -1.65815 |
| Total Torsional Energy | -2.80121 | -3.01580 | -1.33293 | -0.57897 |
| Total Out-of-plane Energy | 0.01462 | 0.02105 | 0.01265 | 0.00795 |
| Total Van der Waals Energy | 12.86332 | 12.39468 | 13.71913 | 11.04721 |
| Total Electrostatic Energy | 13.01431 | 14.24191 | 5.11881 | 5.14934 |
| Total Total Energy | 55.40066 | 58.26956 | 50.85154 | 43.98381 |
Analysis
As can be seen from table 1, exo dimer 1 has lower energy barrier than endo dimer 2 which indicating a more stable dimer; however, endo dimer 2 is the one that produced from cyclodimerisation. Therefore, the cyclodimerisation reaction is kinetically controlled. This could be explained by the smaller torsional and angle bending energies of the endo dimer. Endo dimer has more deviation from the ideal hybridisation angles for the carbon atom. (120o for sp2; 109.5o for sp3); thus, the transition state is more stable than exo dimer.
Similarly, molecule 4 has lower energy barrier than molecule 3, indicating a more stable form; thus it should be the main product if the hydrogenation reaction is thermodynamically controlled.
Intermediate 9 and 10 are atropisomerism[1]because the only distinction between them is the position of the carbonyl group due to highly steric demand removing the free rotation.
In order to rationalise which of the two atropisomers is the more stable, their geometries have been optimised using the same approach as above. In addition, to explore why the functionalization of the alkene is abnormally slow, the parent hydrocarbons of both intermediates have also been optimised. Compare the minimal energies of the hydrocarbon with the alkene to see if alkene is more stable. (reference.)
The rotatable models of intermediate 9(up left), 10(up right) and their parent hydrocarbons (9 bottom left; 10 bottom right) are displayed below.


Pentahelicene |
Pentahelicene |
Pentahelicene |
Pentahelicene |
| Molecules | 9(kcal/mol) | 10(kcal/mol) | 9 Parent Hydrocarbon(kcal/mol) | 10 Parent Hydrocarbon(kcal/mol) |
|---|---|---|---|---|
| Total Bond Stretching Energy | 7.57840 | 7.61512 | 7.57840 | 6.23638 |
| Total Angle Bending Energy | 28.31119 | 19.25446 | 28.31119 | 24.81294 |
| Total Stretch bending Energy | -0.04689 | -0.13199 | -0.04689 | 0.39424 |
| Total Torsional Energy | 0.62478 | 0.55664 | 0.62478 | 9.79062 |
| Total Out-of-plane Energy | 0.99288 | 0.88226 | 0.99288 | 0.03668 |
| Total Van der Waals Energy | 32.91803 | 32.85044 | 32.91803 | 30.78822 |
| Total Electrostatic Energy | 0.32176 | -0.02762 | 0.32176 | 0.00000 |
| Total Total Energy | 70.70016 | 60.99931 | 79.78423 | 72.05907 |
Analysis
Comparing the total energies of intermediate 9 and 10, the conclusion is that intermediate 10 which carbonyl group pointing inward has more stability. This is mainly due to the angle bending energy of the six-membered ring. Intermediate 10 has less deviation from the ideal hybridised angles so it has less bending energy resulting in a more stable form.
Moreover, both parent hydrocarbons of intermediate 9 and 10 have higher total energy than the alkenes. Thus, alkenes are thermodynamically stable than their parent hydrocarbons, and the functionalization becomes quite slow and unfavourable.
Reference
- ↑ See J. G. Vinter and H. M. R. Hoffman, J. Am. Chem. Soc., 1974, 96, 5466–5478 (DOI:10.1021/ja00824a025 DOI:10.1021/ja00824a025 )
Spectroscopic Simulation using Quantum Mechanics
A practice molecule: Spectroscopy of an intermediate relayed to the synthesis of Taxol
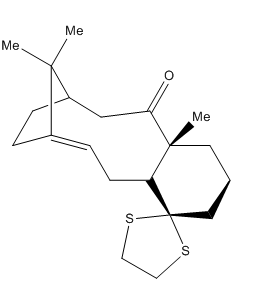
Molecule 17 is called Taxol in life. It is mainly used as an anti-cancer chemotherapy drug. It is originally isolated from the Pacific yew tree, and recently has been found for clinical treatment.[1]
It was drawn by Chemdraw shown in figure on the right and optimized to the minimum energy by Avogadro under the MMFF94s force field. Then, in order to calculate the geometry at the density functional level (DFT), Gaussian was used to generate the NMR spectra for both 1H and 13C, and TMS B3LYP/6-31G(d,p) Chloroform was selected to perform 1H and 13C NMR spectra. In addition, all chemical shifts have been rounded to four decimal places.
Moreover, the chemical shifts for 1H on the methyl groups are the average shift of three protons; for 13 C NMR, chemical shift for the carbons which are adjacent to sulfur atoms should be corrected -3 ppm [2]since there is spin-orbital coupling.
Since the cyclohexane could have different conformations such as chair, boat or twist. The geometry has been optimized to have as low energy as possible. However, this could not be sure, and might also be one of the reasons that the computated results do not match the literature.
Pentahelicene |


| 1H NMR | ' | ' | 13C NMR | ' | ' |
|---|---|---|---|---|---|
| Chemical Shift(ppm) | Degeneracy | Atoms | Chemical Shift(ppm) | Degeneracy | Atoms |
| 5.1494 | 1.0000 | 26 | 216.1036 | 1.0000 | 11 |
| 3.3065 | 1.0000 | 44 | 145.1271 | 1.0000 | 2 |
| 3.2195 | 1.0000 | 43 | 124.6948 | 1.0000 | 3 |
| 3.1571 | 1.0000 | 34 | 90.6461 | 1.0000 | 12 |
| 3.0132 | 2.0000 | 42,41 | 60.6387 | 1.0000 | 9 |
| 2.7136 | 2.0000 | 25,32 | 57.0487 | 1.0000 | 10 |
| 2.6340 | 1.0000 | 45 | 52.4783 | 1.0000 | 6 |
| 2.3952 | 4.0000 | 27,28,35,33 | 51.5468 | 1.0000 | 7 |
| 2.2718 | 2.0000 | 24,30 | 46.6951 | 1.0000 | 4 |
| 2.1201 | 2.0000 | 39,31 | 45.9518 | 1.0000 | 8 |
| 1.9525 | 1.0000 | 36 | 42.1720 | 1.0000 | 13 |
| 1.9022 | 1.0000 | 37 | 40.5727 | 1.0000 | 17 |
| 1.7434 | 1.0000 | 53 | 35.3236 | 1.0000 | 8 |
| 1.5939 | 3.0000 | 29,38,49 | 31.0102 | 1.0000 | 5 |
| 1.4879 | 1.0000 | 40 | 29.3391 | 2.0000 | 15,23 |
| 1.1627 | 1.0000 | 51 | 27.0920 | 1.0000 | 1 |
| 0.9057 | 3.0000 | 48,47,50 | 26.4020 | 1.0000 | 12 |
| 0.8223 | 1.0000 | 46 | 22.9039 | 1.0000 | 14 |
| 0.5903 | 1.0000 | 52 | 19.7265 | 1.0000 | 21 |
Analysis
Compare the calculated chemical shifts against the literature values, 1H NMR spectra has larger chemical shifts than the literature. Generally, the experimental result does not quite match the literature. The proton 52 as well as the protons in the methyl groups are more deshielded in the computated analysis.
And the 13 C NMR has little differece between the calculated and the literature values. A difference table is shown below.
| Literature Value (ppm) | Calculated Value (ppm) |
|---|---|
| 218.79 | 216.1036 |
| 144.63 | 145.1271 |
| 125.33 | 124.6948 |
| 72.88 | 90.6461 |
| 56.19 | 60.6387 |
| 52.52 | 57.0487 |
| 48.50 | 52.4783 |
| 46.80 | 51.5468 |
| 45.76 | 46.6951 |
| 39.80 | 45.9518 |
| 38.81 | 42.1720 |
| 35.85 | 40.5727 |
| 32.66 | 35.3236 |
| 28.79 | 31.0102 |
| 28.29 | 29.3543 |
| 26.88 | 29.3391 |
| 25.66 | 27.0920 |
| 23.86 | 26.4020 |
| 20.96 | 22.9039 |
| 18.71 | 19.7265 |
Analysis
For 13 C NMR, Generally the computated results match the literature values; but most of the chemical shifts are larger than the literature values. First of all, the solvents are different which we use CHCl3 whereas the literature uses C6D6. Also, the temperature is also different. However, it is hard to compare1H NMR because there is many multiplet between 2.80ppm and 1.30 ppm. It is not comparable with our computated results.
The thermodynamic quantities of the Molecule 17 were summarised as below:
| Terms | Energy (Hartree/Particle) |
|---|---|
| Zero-point correction | 0.468008 |
| Thermal correction to Energy | 0.489496 |
| Thermal correction to Enthalpy | 0.490440 |
| Thermal correction to Gibbs Free Energy | 0.421217 |
| Sum of electronic and zero-point Energies | -1651.414399 |
| Sum of electronic and thermal Energies | -1651.392912 |
| Sum of electronic and thermal Enthalpies | -1651.391967 |
| Sum of electronic and thermal Free Energies | -1651.461191 |
Reference
- ↑ K. C. NICOLAOU*†, Z. YANG*, J. J. LIU*, H. UENO*, P. G. NANTERMET*, R. K. GUY*, C. F. CLAIBORNE*, J. RENAUD*, E. A. COULADOUROS*, K. PAULVANNAN* & E. J. SORENSEN*†, Total synthesis of taxol,Nature 367, 630 - 634 DOI:10.1038/367630a0
- ↑ Steven G. Smith and Jonathan M. Goodman,Assigning the Stereochemistry of Pairs of Diastereoisomers Using GIAO NMR Shift Calculation,2009, 74 (12)DOI:10.1021/jo900408d
- ↑ L. Paquette, N. A. Pegg, D. Toops, G. D. Maynard, R. D. RogersJ. Am. Chem. Soc. , 1990, 112, 277-283. DOI:10.1021/ja00157a043
Part Two
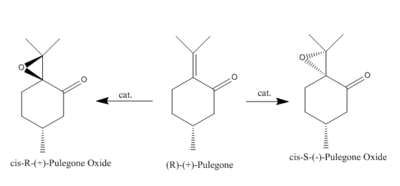
The Crystal Structures of Shi and Jacobsen Catalyst

The structures of Shi's and Jacobsen catalyst have been drawn by Chemdraw 3D, and shown in Figure 7. Both of the them are used in asymmetric epoxidation of alkenes. In order to understand more about the catalysts' properties, Conquest and Mercury program have been used,and the crystal structures of Shi's catalyst and Jacobsen catalyst are displayed below.
Pentahelicene |
Pentahelicene |
Shi Catalyst
The two C-O bonds attached to one anomeric center have different bond lengths. One is shorter than the covalent radii (142 pm) whereas the other one is longer. Since there is a lone pair of electron on the oxygen atom, the donation of the electrons would shorten one of the C-O bonds and lengthen the other one as there is more electron density in the anti-bonding orbital. (C-O σ * orbital) Also, since the carbonyl group is electron-withdrawing, it will determine which C-O bond to be shortened. More information is illustrated in the figure on the right.
One interesting fact in this analysis is that Mercury generates two crystal structures of the Shi catalyst at the same time; however, theses two structures are not identical because the shortened C-O bond is different between these two as well as the bond length.
Jacobsen Catalyst
Jacobsen catalyst acquires a square based pyramidal conformation with the chloride in the axial position while other ligands are in the equatorial positions. The reason is that equatorial ligands are all attached to a ring structure; therefore, in order to minimize the torsional energy, it is better for them to sit as plane as possible; as can be seen from the diagram, the four ligands are almost co-planar. Moreover, the t-butyl groups are ortho to each other which would also result in a lower energy for the transition state.
The Calculated NMR Properties of the Epoxides

In order to analyze the NMR spectra,geometries were optimised using the same approach as described for Taxol and the results are listed below.
Pentahelicene |
Epoxide 2
Pentahelicene |
Epoxide 3

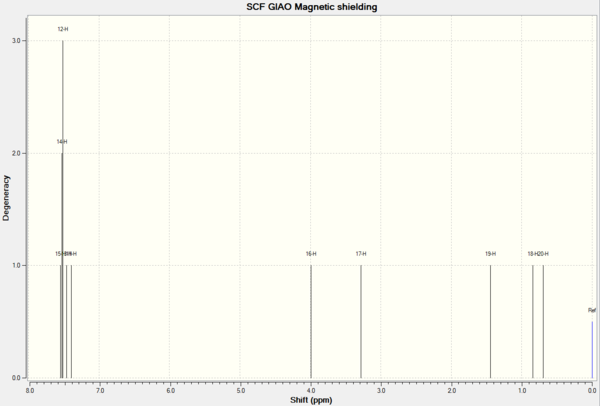
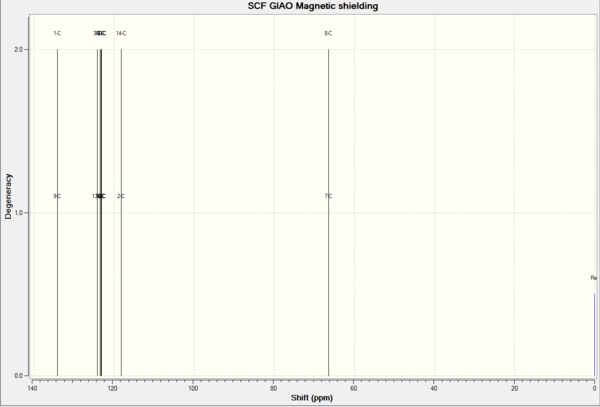

| 13C NMR Epoxide 2 | ' | ' | 1H NMR Epoxide 2 | ' | ' |
|---|---|---|---|---|---|
| Chemical Shift(ppm) | Degeneracy | Atoms | Chemical Shift(ppm) | Degeneracy | Atoms |
| 132.8144 | 1.0000 | 1 | 7.5287 | 3.0000 | 15,14,12 |
| 123.4080 | 1.0000 | 5 | 7.4564 | 1.0000 | 13 |
| 123.0794 | 1.0000 | 3 | 7.3927 | 1.0000 | 11 |
| 122.7152 | 1.0000 | 6 | 3.9887 | 1.0000 | 16 |
| 122.4430 | 1.0000 | 4 | 3.2811 | 1.0000 | 17 |
| 121.3628 | 1.0000 | 2 | 1.4411 | 1.0000 | 19 |
| 58.9985 | 1.0000 | 7 | 0.8392 | 1.0000 | 18 |
| 57.6573 | 1.0000 | 8 | 0.6922 | 1.0000 | 20 |
| 13.0691 | 1.0000 | 9 |
| 13C NMR Epoxide 3 | ' | ' | 1H NMR Epoxide 3 | ' | ' |
|---|---|---|---|---|---|
| Chemical Shift(ppm) | Degeneracy | Atoms | Chemical Shift(ppm) | Degeneracy | Atoms |
| 134.0895 | 2.0000 | 9,1 | 7.5705 | 2.0000 | 26,17 |
| 124.2212 | 2.0000 | 13,3 | 7.4791 | 8.0000 |
20,23,19,24,25,18,27,16 |
| 123.5182 | 2.0000 | 5,11 | 3.5378 | 2.0000 | 22,21 |
| 123.2125 | 2.0000 | 12,4 | |||
| 123.0772 | 2.0000 | 6,10 | |||
| 118.2631 | 2.0000 | 2,14 | |||
| 66.4260 | 2.0000 | 7,8 |
Analysis
It would be helpful if compare with literature values because it is not confirmed that the epoxides have been optimised to the lowest energy.
The Assignment of the Absolute Configurations for Alkenes 2 and 3
The epoxidation of alkenes using either catalysts is stereospecific;thus, both R,R and S,S species could be the product. In order to identify which species is produced, optical rotatory powers have been investigated.
The reported literature for optical rotations
Literature values of the optical rotatory power for SS and RR epoxides have been found out using Reaxys and listed in the following table.
| Epoxide | 2 S,S [1] | 2 R,R [2] | 3 S,S [3] | 3 R,R |
|---|---|---|---|---|
| Concentration (g/100ml) | 1 | 0.32 | 0.56 | 0.73 |
| Enantiometric Excess (%) | 99 | 90 | 89 | 97 |
| Solvent | Chloroform | Chloroform | Chloroform | Chloroform |
| Optical Rotation | -41.8o | 44.3o | -205.2o | 334.6o |
| Wavelength | 589 nm | 589 nm | 589 nm | 589 nm |
| Temperature | 25oC | 25oC | 20oC | 25oC |
The calculated chiroptical properties of the product
Next, Avogadro has been used to optimized the geometry of the epoxides, then Gaussian was used to calculate the optical rotatory power of both RR and SS epoxides under CAM-B3LYP Method, 6-311++g(2df,p) Basis between 589 nm and 365 nm, and chloroform was used as the solvent. The results are listed in the following table.
| Epoxide 2 S,S | Epoxide 2 R,R | Epoxide 3 S,S | Epoxide 3 R,R | |
|---|---|---|---|---|
| αd at 589 nm | -54.67o | 46.77o | -302.38o | 298.28o |
Analysis
Comparing the literature values with the computated values, the conclusion is that although the magnitude of the results were not quite match up, the signs still match each other. Also the difference in magnitude is acceptable. Hence, the literature values are reasonable. The origin of the difference might be caused by different temperature and different concentration. But overall, the literature gives a reliable value.
Using the calculated properties of transition state for the reaction
In order to identify which of the RR or SS species epoxides would be more likely to be produced, free energies of the transition states for Shi epoxidation of β-methyl styrene have been calculated in the following table. Gibbs free energy is calculated by the formula G = ΔG= -RTlnK.
| R,R Free Energies(Hartrees) | S,S Free Energies(Hartrees) | |
|---|---|---|
| TS 1 | -1343.02297 | -1343.017942 |
| TS 2 | -1343.019233 | -1343.015603 |
| TS 3 | -1343.029272 | -1343.023766 |
| TS 4 | -1343.032443 | -1343.024742 |
| Average Free Energy | -1343.02598 | -1343.020513 |
| Free Energy Difference between RR and SS | -0.00546625 | |
| K Value | 326.9 | |
| Enantiomeric Excess (RR-SS) | 99.4 |
Analysis
All of the four transition states of RR species are more stable than SS species; thus, RR epoxide would be the main product from the epoxidation of β-methyl styrene. In addition, this enatiomeric excess (99.4) is quite similar to the literature value (99) in table 8; hence, the literature calculation is again confirmed to be reliable.
Further Analysis
ECD which is the electronic circular dichroism has not been done because there is no appropriate chromophore for the epoxides; VCD which is the vibrational circular dichorism has been done for both SS and RR epoxides to investigate more about the absolute configuration of the epoxides.


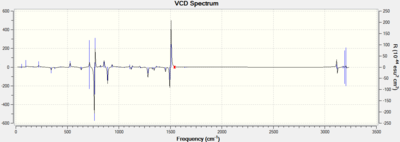

Analysis
Clearly, the VCD spectra of the RR and SS epoxides are just mirror images of each other by the x-axis. This result match the definition of enantiomers since they have opposite vibrational signs. Further analysis of VCD spectra would help to understand the absolute configuration of epoxides, but there is no appropriate instrument in the department.
Reference
- ↑ Hui Lin, Yan Liu, Zhong-Liu Wu, Tetrahedron: Asymmetry 2011, 22, 134 DOI:10.1016/j.tetasy.2010.12.022
- ↑ Wong, O. A.; Wang, B.; Zhao, M.-X.; Shi, Y. Journal of Organic Chemistry 2009, 74, 6335 DOI:10.1021/jo900739q
- ↑ Niwa, T.; Nakada, M. Journal of the American Chemical Society 2012, 134, 13538DOI:10.1021/ja304219s
- ↑ Wong, O. A.; Wang, B.; Zhao, M.-X.; Shi, Y. Journal of Organic Chemistry 2009, 74, 6335 DOI:10.1021/jo900739q
NCI Analysis for the Transition State
The first transition state for Shi epoxidation of R,R-trans-beta-methyl styrene was selected to undergo the NCI analysis. And the rotatable model is displayed below.
Orbital |
Non-covalent interactions reveals the properties of the electron density and its curvatures. As can be seen from the model above, the colours demonstrate whether the interaction is attractive or repulsive. The green region in the middle of the diagram indicates that the interaction between the active species of catalyst and the substrate is mildly attractive. Oxygen atoms on the active species of catalyst are labelled in pink; thus, it can be concluded that in order to have the R,R stereoselectivity, the two adjacent oxygen atoms (O6 &O8) have to be connected to the methyl group of the alkene whereas the single oxygen (O10)has to be connected with the phenyl group of the alkene.
QTAIM Analysis for the First Transition State of R,R Series of Shi Epoxidation
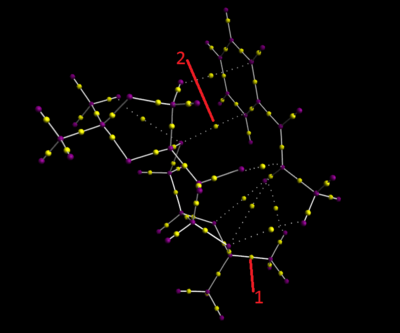
QTAIM analysis in the active site of the reaction transition state is complementary to the NCI analysis. It focuses on both strong interactions which are bond as well as weak interactions that are identified in the NCI analysis. The bond topological critical points (BCPs) are indicated as the yellow spheres along the straight line between two nuclei. Arrow 2 identifies BCPs with are strong interactions which are bonds. While arrow 1 also indicates a BCP but it is a weak non-covalent one.
One of the interesting BCPs is that there is a carbon on the substrate which has three non-covalent bonds attached to the active species of the catalyst.
Suggesting new candidates for investigations
By searching in Reaxys, two new epoxides and their corresponding alkene have been found and shown in the figure 21. The specific information about the alkene has been found in the catalogue of Sigma-Aldrich: CAS number 89-82-7 and product number 376388.
The optical rotation properties of the two new epoxides are demonstrated in the table 11.
| Terms | R-species | S-species |
|---|---|---|
| Concentration (g/100ml) | 0.03 | 0.03 |
| Wavelength (nm) | 324 | 327 |
| Solvent | Ethanol | Ethanol |
| Temperature | 25oC | 25oC |
| αd | 853.9o | -1177.9o |
Reference
- ↑ Reusch; Johnson Journal of Organic Chemistry 1963, 28, 2557 DOI:10.1021/jo01045a016
Pros and Cons of Softwares
At the end of the compuational experiment, it is vital to understand the merits and demerits of the softwares that have been used; hence, it would help us to choose the more appropriate software for future analysis.
Pros
Chembio 3D: Sometimes only Chembio 3D could draw the appropriate structures of molecules when Chemdraw sometimes changes the stereochemistry weirdly.
Gaussview: Could be used for analysing NMR, UV and IR spectra.
Avogadro: Could be used for optimizing the geometry of molecule and obtaining the minimum energy.
Conquest & Mercury: Easy to use for measuring the bond length
Cons
ChemBio 3D: Takes long time for optimisation than Avogadro and does not have the MMFF94s force field.
Gaussview: Slow software; the same file cannot be opened in Gaussview on one computer but maybe opened on a laptop.
Avogadro: QTAIM analysis has to be run in MacBook instead of Windows; crashes often
