Rep:Mod:anguswiki
NH3 molecule
- N-H Bond distance:1.01798 Angstrom
- H-N-H Bond angle: 105.7412 Degree
- Calculation method: RB3LYP
- Basis set: 6-31G(d,p)
- Final energy E(RB3LYP): -56.55776873 a.u.
- RMS gradient: 0.00000485 a.u.
- Dipole moment: 1.8466 Debye
- Point group of your molecule: C3V
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES
Rotatable 3d images of optimized NH3
Link
File:ANGUS PHUNT NH3 OPTF POP.LOG
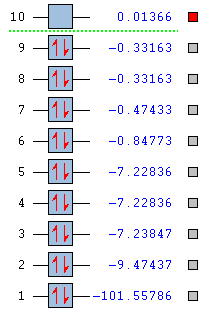
Vibrational analysis of NH3

(1)How many modes do you expect from the 3N-6 rule?
6 modes
(2)Which modes are degenerate (ie have the same energy)?
Vibrational modes 2 and 3, 5 and 6 are degenerate as their stretching frequencies are the same.
(3)Which modes are "bending" vibrations and which are "bond stretch" vibrations?
Bending: Vibrational modes 1,2,3
Stretching: Vibrational modes 4,5,6
(4)Which mode is highly symmetric?
Vibrational mode 4
(5)One mode is known as the "umbrella" mode, which one is this?
Vibrational mode 1
(6)How many bands would you expect to see in an experimental spectrum of gaseous ammonia?
2 bands are expected to see in the experimental spectrum of NH3. Although there are 6 vibrational frequency calculated according to GaussView,vibrational modes 2 and 3, 5 and 6 are degenerate. So 4 bands should be observed. However, vibrational modes 4,5 and 6 are of very low intensity comparing to the peak intensity according to the table. They can not be observed.
Charges on NH3
- Charge on nitrogen: -1.125 a.u.
- Charge on each hydrogen: 0.375 a.u.
The electronegativities of N and H are 3.04 and 2.1 respectively. As nitrogen atom is more electronegative than hydrogen atom, nitrogen atom tends to attract electrons to itself in a covalent bond. Therefore, negative charge is expected on nitrogen atom and positive charge is expected on the three hydrogen atoms.
H2 molecule
- H-H Bond distance:0.74279 Angstrom
- Calculation method: RB3LYP
- Basis set: 6-31G(d,p)
- Final energy E(RB3LYP): -1.17853936 a.u.
- RMS gradient: 0.00000017 a.u.
- Point group of your molecule: D*H
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES
Rotatable 3d images of optimized H2
Link
File:ANGUS PHUNT H2 OPTF POP.LOG
Vibrational analysis of H2

N2 molecule
- N-N Bond distance:1.110550 Angstrom
- Calculation method: RB3LYP
- Basis set: 6-31G(d,p)
- Final energy E(RB3LYP): - 109.52412868 a.u.
- RMS gradient: 0.00000060 a.u.
- Point group of your molecule: D*H
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
Rotatable 3d images of optimized N2
Link
File:ANGUS PHUNT N2 OPTF POP.LOG
Vibrational analysis of N2

Literature values of vibrational frequency of N2
- There is a difference between the value obtained in Gaussview and the literature value. The frequency obtained in Gaussview is at 0 Kelvin but not for the literature values. The lower the temperature is, the lower the stretching frequency is.
- Value obtained using GaussView: 2457 cm-1
- Literature value: 2863 cm-1[1]
- ↑ Barnes, A. Journal of Molecular Structure 1980, 60, 343–346.
Haber-Bosch process analysis
- E(NH3)= -56.557769 a.u.
- 2*E(NH3)= -113.115538 a.u.
- E(N2)= -109.524129 a.u.
- E(H2)= -1.178539 a.u.
- 3*E(H2)= -3.535618 a.u.
- ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -146.48 kJ/mol
- As ΔE is negative(exothermic), which means that the ammonia product is in lower energy than the gaseous reactants. Therefore, the ammonia product is more stable.
HCl molecule
- H-Cl Bond distance:1.28599 Angstrom
- Calculation method: RB3LYP
- Basis set: 6-31G(d,p)
- Final energy E(RB3LYP): -460.80077875 a.u.
- RMS gradient: 0.00005211 a.u.
- Dipole moment:1.4334 Debye
- Point group of your molecule: C*V
Item Value Threshold Converged? Maximum Force 0.000090 0.000450 YES RMS Force 0.000090 0.000300 YES Maximum Displacement 0.000139 0.001800 YES RMS Displacement 0.000197 0.001200 YES
Rotatable 3d images of optimized HCl
Link
File:ANGUS PHUNT HCL OPTF POP.LOG
Vibrational analysis of HCl

Literature values of vibrational frequency of HCl
- There is a difference between the value obtained in Gaussview and the literature value. The frequency obtained in Gaussview is at 0 Kelvin but not for the literature values. The lower the temperature is, the lower the stretching frequency is.
- Value obtained using GaussView: 2957 cm-1
- Literature value: 2818 cm-1[1]
- ↑ Barnes, A. Journal of Molecular Structure 1980, 60, 343–346.
Charges on HCl
- Charge on chlorine: -0.284 a.u.
- Charge on hydrogen: 0.284 a.u.
The electronegativities of Cl and H are 3.16 and 2.1 respectively. As chlorine atom is more electronegative than hydrogen atom, chlorine atom tends to attract electrons to itself in a covalent bond. Therefore, negative charge is expected to be on the chlorine atom and positive charge is expected on the hydrogen atom. As HCl is a diatomic molecule, the two charges are equal but opposite.
Moleuclar Orbital Analysis of HCl