Rep:Mod:ameliatayloryoung
Introduction
A Potential Energy Surface (PES) is a 3D representation of the potential nuclei energy if it's position is allowed to move. It appears as a landscape of valleys and peaks. Using the positions of the nuclei we can then calculate the energy of a given molecule. A molecule can be optimized to a minimum at which it is chemically stable, i.e. reactants and products. At this point the gradient is zero and there is an energy rise in all directions. A molecule can be optimized to a transition structure, this is a maxima in the PES. The gradient is also zero but the energy decreases in the reaction path, this is known as a saddle point. From the first derivative these stationary points can be found, the second derivative must be calculated to determine the curvature, i.e. whether the point is a minimum or a maximum. The second derivative (or the force constants) is found from a frequency calculation.
Nf710 (talk) 15:44, 4 November 2016 (UTC) Good understanding, would have been nice to see some knowledge of the methods included here.
Exercise 1:
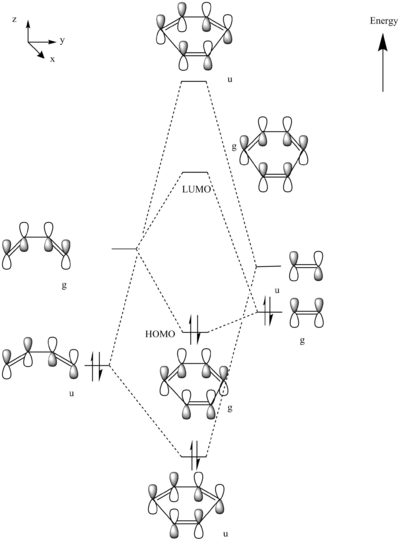
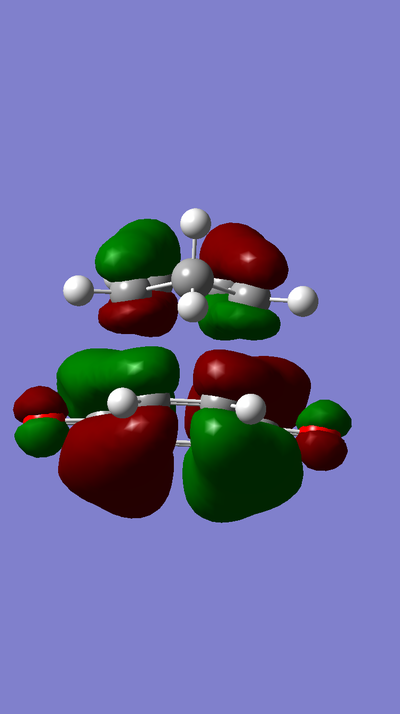
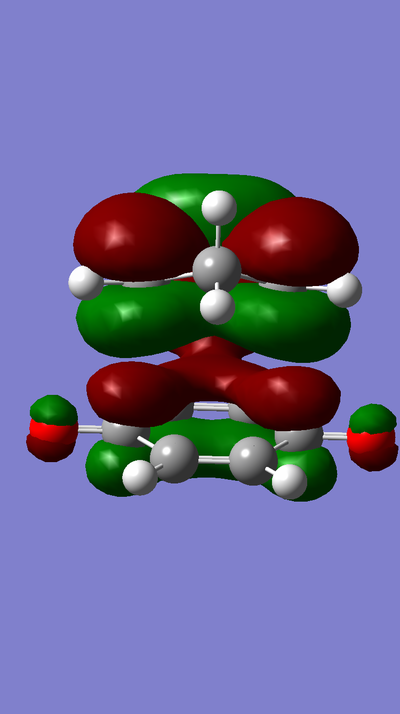
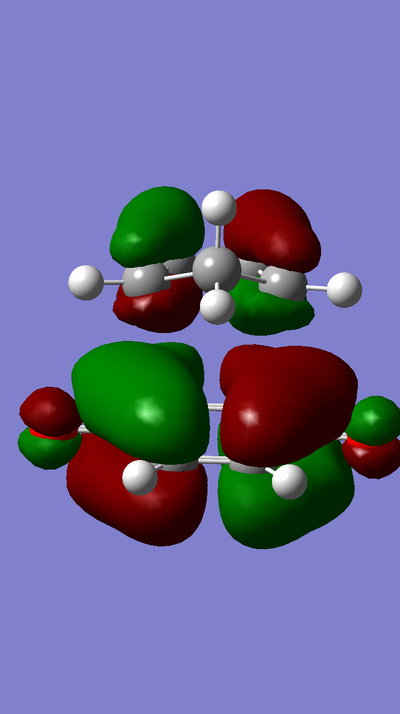
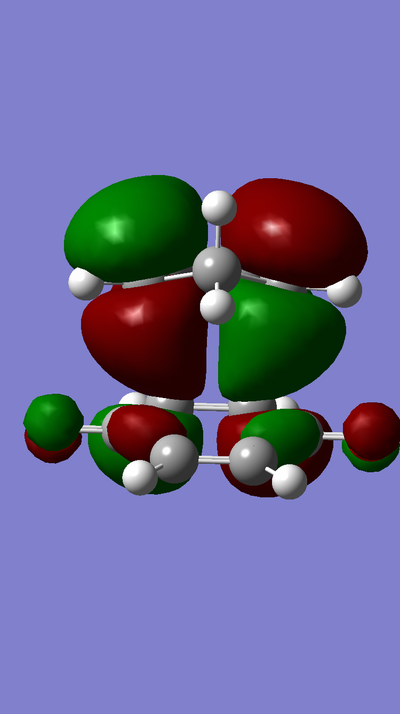
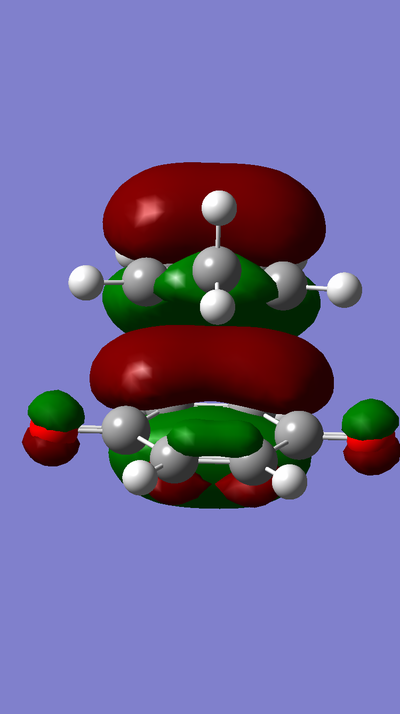
MO Diagram:
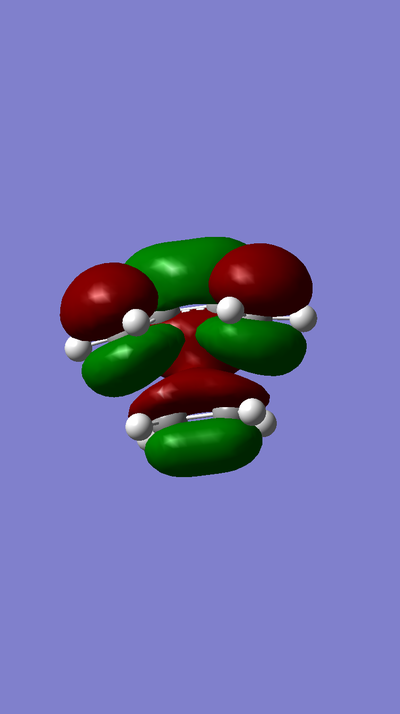
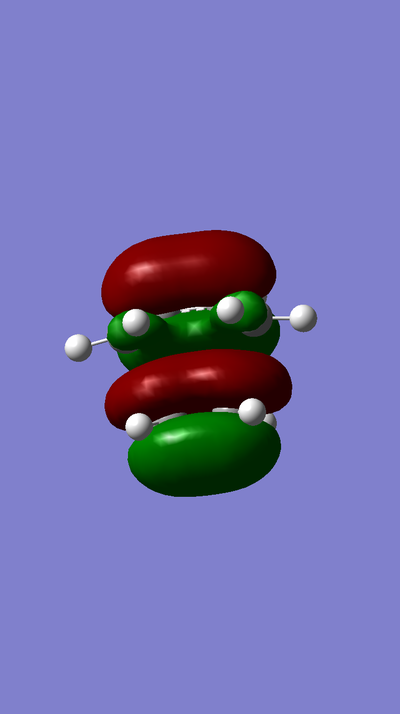
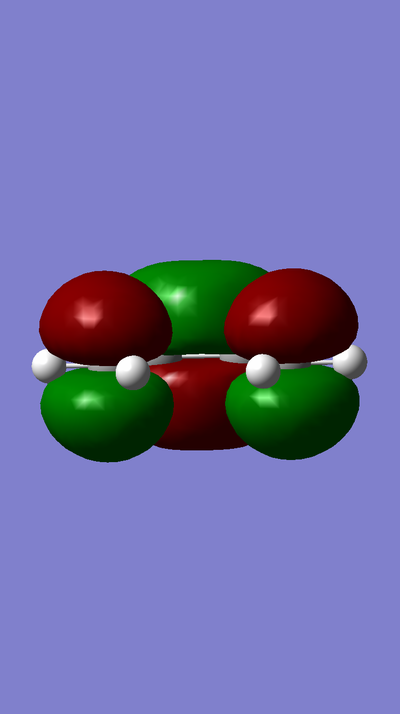
MOs:
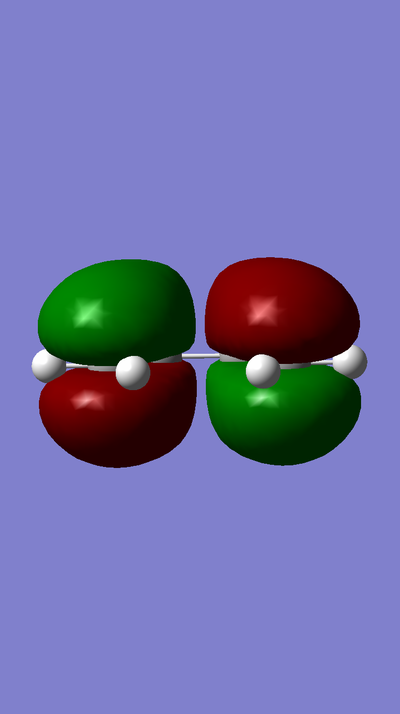
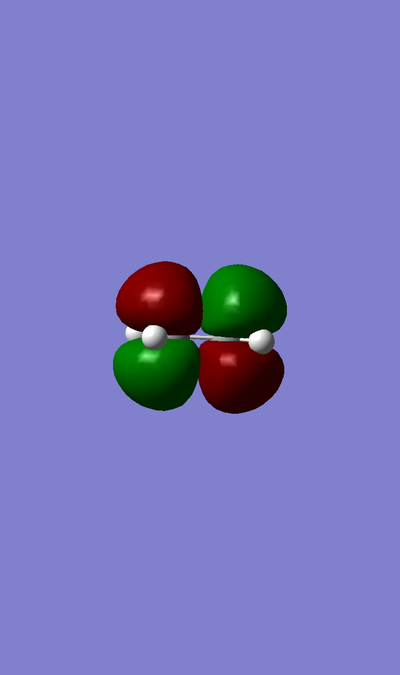
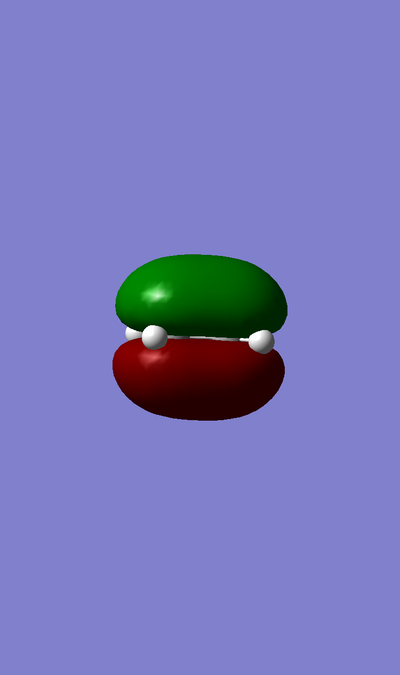
MO of LUMO of TS:
MO of HOMO of TS:
MO of LUMO of Butadiene:
MO of HOMO of Butadiene:
MO of LUMO of Ethene:
MO of HOMO of Ethene:
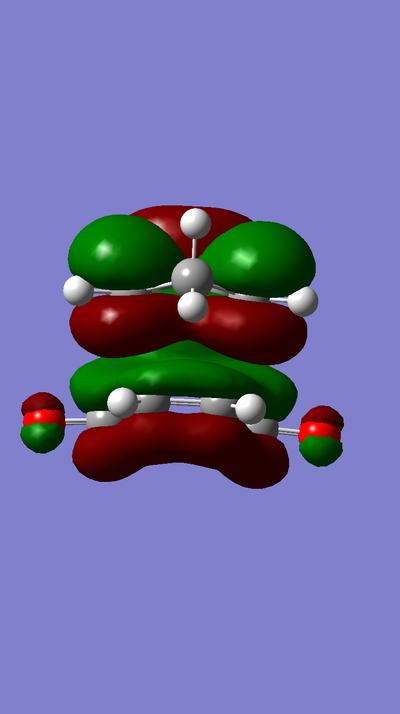
The HOMO of Ethene interacts with the LUMO of Butadiene to form the fully occupied HOMO and the LUMO of the TS, as depicted in the MO diagram file.
The LUMO of Ethene interacts with the HOMO of Butadiene to form the lowest and highest energy MOs in the diagram. The lowest energy MO is fully occupied, whereas the highest energy MO is unoccupied.
For an interaction to be allowed there must be a change in parity, i.e gerade to ungerade and vice versa. If there is no change in parity the reaction is disallowed. In the case of a gerade-ungerade interaction the orbital overlap integral will be zero. The orbital overlap integral will be non-zero for a gerade-gerade interaction and an ungerade-ungerade interaction.
Nf710 (talk) 15:50, 4 November 2016 (UTC) This answer is contradicting your MO diagram where symmetry is conserved.
Table showing measurments of C-C bonds:
| Reaction Progress | Reactant | TS | Product | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bond | Ethene | Butadiene = | Butadiene - | Ethene | Butadiene = | Butadiene - | New | = | - | - | - |
| Bond Length (Angstroms) | 1.33 | 1.34 | 1.46 | 1.38 | 1.38 | 1.41 | 2.11 | 1.33 | 1.50 | 1.53 | 1.54 |
From the reactants to the TS, both the ethene and the butadiene double bonds lengthen as they transition to single bonds (from sp2 to sp3). The single bond in butadiene shortens as it transitions to a double bond. From the TS to the product the single bond of butadiene has shortened to the length of a double bond. Two single bonds have formed between the two fragments, which were initially 2.11 Angstroms apart. The single bonds in the product have lengthened from double bonds now that he carbons are sp3 hybridised. A typical sp3-sp3 C-C bond length is 154pm compared to 147pm for an sp2-sp2 C-C bond.[1]. The Van der Waals radius for a carbon atom is 1.7 Angstroms.[2] The length of the partially formed new C-C bond is smaller than twice the Van der Waals radius, showing that there is some interaction between the carbon atoms.
Illustration of vibration corresponding to reaction path at the transition state:
The lowest positive frequency is a rotation rather then a stretching motion, therefore this frequency doesn't have any significance in the transition from reactants to products. The formation of the two new bonds is synchronous, i.e they form in the same step.
Nf710 (talk) 15:56, 4 November 2016 (UTC) You do everything required, but didn't go into a huge amount of effort. however good effort.
Exercise 2:
MOs:
Endo TS:
Highest energy MO:
MO of LUMO:
MO of HOMO:
Lowest energy MO:
Exo TS:
Highest energy MO:
MO of LUMO:
MO of HOMO:
Lowest energy MO:
(Fairly difficult to compare these images. It would be better to place them in a table and/or shrink them Tam10 (talk) 11:53, 31 October 2016 (UTC))
I would expect a normal energy demand from this reaction seeing as cyclopentadiene is electron rich and benzoquinone is electron poor. The strongest interaction is from the MOs of gerade symmetry as a result of the interaction between the LUMO of the diene and the HOMO of the dienophile. These FOs are closer in energy compared to the HOMO of the diene and the LUMO of the dienophile. The energies of the lowest energy MO and the HOMO are almost degenerate, considering PM6 was used these MOs cannot be distinguished. This result shows there is an inverse electron-demand.
Table showing energies:
| Endo | Exo | |||||
|---|---|---|---|---|---|---|
| Reactants | TS | Product | Reactants | TS | Product | |
| Sum of electronic and thermal free energies | 0.130704 | -575.383855 | 0.108078 | 0.130697 | -575.381307 | 0.109351 |
| Reaction Barrier | -575.514704 | -575.511697 | ||||
| Reaction Energy | -0.022626 | -0.021346 |
(You've mixed PM6 energies and B3LYP energies. If you converted the energies to kj/mol or spotted the negative activation energy you should be able to tell that something has gone wrong - you're showing an activation energy of -1.5MJ/mol Tam10 (talk) 11:53, 31 October 2016 (UTC))
The reaction barrier of the endo-configuration is of a lower energy. The TS is reached quicker confirming that this is the kinetic product. The reaction energy of the exo-configuration is lower, confirming this is the thermodynamic product. In the endo TS confirmation there are secondary orbital interactions possible between the alkene C=C pi* orbitals and the C=O pi* orbitals. These interactions lower the energy of the endo TS, consequently the endo product is kinetically favourable. There are only primary orbital interactions possible in the exo TS confirmation. However there are fewer steric clashes in the exo conformation. Consequently the exo conformation is the thermodynamic product.
Nf710 (talk) 16:06, 4 November 2016 (UTC) Your TS are correct so you have been rewarded there but I can award you for your reasoning of the kinetic and thermo products because you mixed up the energies.
Exercise 3:
Reaction Coordinates:
Diels Alder:
Exo
Endo
Cheletropic:
Exo
Endo
The Cheletropic reaction does not proceed in the endo-conformation due to the energy of the transition state being too high.
Table showing energies for Diels Alder Reaction:
| Endo | Exo | |||||
|---|---|---|---|---|---|---|
| Reactants | TS | Products | Reactants | TS | Products | |
| Thermal point corrections | 0.141809 | 0.142192 | 0.145011 | 0.142005 | 0.142126 | 0.145001 |
| Zero point corrections | 0.132249 | 0.131998 | 0.135511 | 0.131987 | 0.131899 | 0.135612 |
| Sum of electronic and thermal energies | 0.131934 | 0.136784 | 0.067003 | 0.140363 | 0.138399 | 0.066033 |
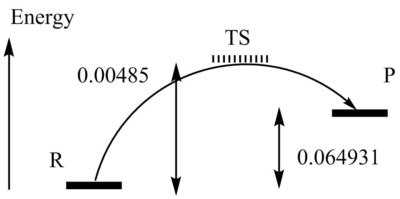
| Activation energy | 0.00485 | -0.001964 | ||||
| Reaction energy | -0.064931 | -0.07433 |
My results show the activation energy of the exo-configuration to be lower and the reaction energy of the endo-configuration to be greater. I would expect the activation energy of the endo reaction to be lower due to secondary orbital overlap interactions. The activation barrier is lower so the TS is formed faster, confirming that this it would be the kinetic product.The reaction energy of the exo reaction is lower due to reduced steric hindrance, confirming that this would be the thermodynamic product.
(You've used the corrections, not the energies themselves Tam10 (talk) 12:04, 31 October 2016 (UTC))
Table showing energies for Cheletropic Reaction:
| Reactants | TS | Product | |
|---|---|---|---|
| Thermal point correction | 0.141869 | 0.141997 | 0.145233 |
| Zero point correction | 0.132451 | 0.131556 | 0.136201 |
| Sum of electronic and thermal energies | 0.116969 | 0.145999 | 0.043588 |
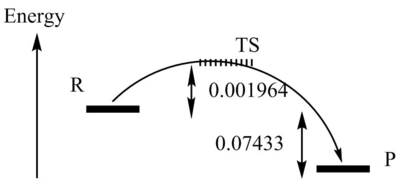
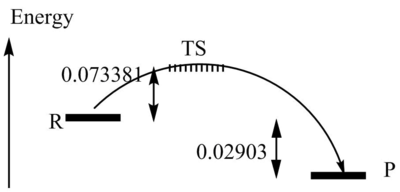
| Activation Energy | 0.02903 | ||
| Reaction Energy | -0.073381 |
My results show the Cheletropic reaction to have the greatest activation energy and the endo reaction to have the greatest reaction energy. The reaction energy of the endo reaction is greater than the activation energy of the cheletropic reaction, consequently this would be the least favourable reaction. I believe the Cheletropic reaction is less favourable due to the formation of a less stable five-membered ring compared to a six-membered ring. And the endo reaction is less favourable due to steric hindrance. The exo configuration is shown to have the lowest reaction energy, due to a lack in steric hindrance, so this route would be the most favoured.
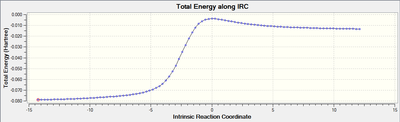
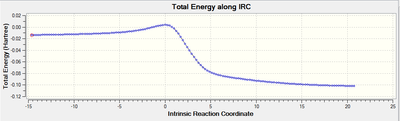
Reaction Profiles and IRC plots:
Diels Alder:
Endo:
Exo:
Cheletropic:
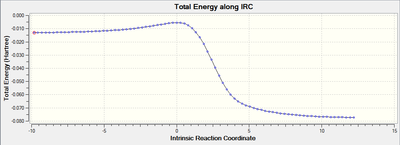
My reaction scheme for the exo-configuration of the diels alder reaction and the cheletropic reaction show a decrease in energy going from the reactants to the products. In the endo-configuration for the diels alder reaction there is an increase in energy from the reactants to the products.
Xylylene is highly unstable. IRCs for the three reactions show two double bonds at the start of the reaction. Around half way through the six-membered ring shows dashed lines across the six carbon atoms representing the delocalised electrons.
Conclusion
In the 4+2 cycloaddition of butadiene with ethlyene, the C-C bond length analysis confirms the formation of two new single bonds between the two fragments and the change in hybridisation within the fragments. Illustration of the vibration corresponding to the reaction path at the TS showed the bond formation to be synchronous. The reaction between electron rich cyclopentadiene and electron poor benzoquinone was expected to be a normal electron demand, however my results showed it to in fact be inverse. I believe this error is due to the inaccuracy of PM6 as method of calculation. Energy calculations confirmed that the endo-configuration is the kinetic product, due to secondary orbital overlap interactions and that the exo-configuration is the thermodynamic product due to sterics. Comparison of Diels Alder and Cheletropic reactions produced results showing the Cheletropic reaction to have the greatest activation energy and the endo-configuration of the diels alder reaction to have the greatest reaction energy. These are due to the lower stability of a five-membered ring compared to a six-membered ring and steric hindrance in the endo-configuration. Using GaussView it was possible to calculate TSs, energy barriers and reaction paths of different structures along the PES, however the method of calculation is significant in the accuracy of results.