Rep:Mod:am8616
NH3 Molecule Report
NH3 Molecular Properties
| Method | Result |
|---|---|
| Molecule | NH3 |
| Calculation | RB3LYP |
| Basis Set | 6-31G(d.p) |
| Energy | -56.55776873 Hartree |
| RMS Gradient | 0.00000485 |
| Point Group | C3V |
| NH Bond Length | 1.01798 Å |
| HNH Bond Angle | 105.741° |
Online Sources had similar values of bond angles[1]
Item Table For Optimised NH3
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES
NH3 Structure
NH3 |
The optimisation file is linked to here
IR Information
| Question | Answer |
|---|---|
| How many modes do you expect from the 3N-6 rule? | 6 modes |
| Which modes are degenerate (ie have the same energy)? | Modes 2 and 3 are degenerate, modes 5 and 6 are also degenerate |
| Which modes are "bending" vibrations and which are "bond stretch" vibrations? | Modes 1, 2 and 3 are bends, Modes 4, 5 and 6 are stretches |
| Which mode is highly symmetric? | Mode 4 |
| One mode is known as the "umbrella" mode, which one is this? | Mode 1 |
| How many bands would you expect to see in an experimental spectrum of gaseous ammonia? | 2 bands would be visible (the 1089.54 peak and the degenerate 1693.95 peaks), however the other 2 Display Vibrations would still be present but their relative intensities are very small so would not be seen. |
The relative intensities of peaks 5 and 6(shown below) can be seen to be much lower than the other stretching and bending peaks.

When searching for IR Values online, sources confirmed our analysis of which peaks are stretch and bending. [2]
Charge of Nitrogen and Hydrogen on a NH3 Molecule
| Atom | Charge |
|---|---|
| Nitrogen | -1.125eV |
| Hydrogen | 0.375eV |
We would expect the nitrogen atom to be more negative than the hydrogen atoms as it is more electronegative. This means the nitrogen will pull electron density towards itself from the hydrogens causing it to be more negative. The sum of all the charge values equals 0 which is what we would expect as NH3 is a neutral molecule.
N2 Molecule Report
N2 Molecular Properties
| Method | Result |
|---|---|
| Molecule | An N2 Molecule |
| Calculation | RB3LYP |
| Basis Set | 6-31G(d.p) |
| Energy | -109.52412868 Hartrees |
| RMS Gradient | 0.00000060 |
| Point Group | DH |
| N2 Bond Length | 1.10550Å |
| N2 Bond Angle | 180° |
Item Table For Optimised N2
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
N2 Structure
N2 |
The optimisation file is linked to here
IR Information
How many modes do you expect from the 3N-6 rule? Only 1 with a frequency of 2457.33, however since N2 is a homonuclear molecule, there will be no overall change in dipole moment when it symmetrically stretches meaning no peak will be observed.

N2 Molecule Charges
As you would expect for a homodinuclear molecule, each individual atom has a charge of 0eV as they have the exact same electronegativity so there's no net displacement of electrons.
H2 Molecule Report
| Method | Result |
|---|---|
| Molecule | H2 |
| Calculation | RB3LYP |
| Basis Set | 6-31G(d.p) |
| Energy | -1.17853936 Hartrees |
| RMS Gradient | 0.00000017 |
| Point Group | DH |
| H2 Bond Length | 0.74279Å |
| H2 Bond Angle | 180° |
Item Table For Optimised H2
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES
H2 Structure
H2 |
The optimisation file is linked to here
IR Information
How many modes do you expect from the 3N-6 rule?
We would expect 1 peak from this rule at a frequency of 4465.68. However H2 is a homonuclear molecule so there will be no overall change in dipole moment when it symmetrically stretches meaning no peak will be observed.

H2 Molecule Charges
As you would expect for a homonuclear molecule, each individual atom has a charge of 0eV as they have the exact same electronegativity as the distribution of electrons is exactly the same on each atom so there's no net charge.
Haber-Bosch Process
Energies
| Enthalpy | Value / Hartree |
|---|---|
| E(NH3) | -56.55776873 |
| 2*E(NH3) | -113.1155375 |
| E(N2) | -109.52412868 |
| E(H2) | -1.17853936 |
| 3*E(H2) | -3.53561808 |
| ΔE=2*E(NH3)-[E(N2)+3*E(H2)] | 0.05579074 |
Converting Hartree to KJ/mol we get a ΔE value of -146.48 KJ/mol.
F2 Molecule Report
F2 Molecular Properties
| Method | Result |
|---|---|
| Molecule | F2 |
| Calculation | RB3LYP |
| Basis Set | 6-31G(d.p) |
| Energy | -199.49825218 Hartree |
| RMS Gradient | 0.00007365 |
| Point Group | DH |
| F2 Bond Length | 1.40281Å |
| F2 Bond Angle | 180° (Linear Molecule) |
Item Table For Optimised F2
Item Value Threshold Converged? Maximum Force 0.000128 0.000450 YES RMS Force 0.000128 0.000300 YES Maximum Displacement 0.000156 0.001800 YES RMS Displacement 0.000221 0.001200 YES
F2 Structure
F2 |
The optimisation file is linked to here
IR Information
How many modes do you expect from the 3N-6 rule?
We would expect 1 peak from this rule at a frequency of 1065.09. However F2 is a homonuclear molecule so there will be no overall change in dipole moment when it symmetrically stretches meaning no peak will be observed.

F2 Molecule Charges
As you would expect for a homonuclear molecule, each individual atom has a charge of 0eV as they have the exact same electronegativity meaning that the electrons will be evenly distributed between the atoms so there will be no net charge.
F2 Molecular Orbitals
This is a list of Fluorine's Bonding, Anti-Bonding and Non-Bonding Molecular Orbitals. The list of numbers on the side gives the Molecular Orbital Energies. As Fluorine has a electronic configuration of 1s22s22p5, picturing a molecular orbital diagram we know that it will have 9 occupied Molecular Orbitals which is indicated by the presence of arrows in the blue boxes representing electrons.
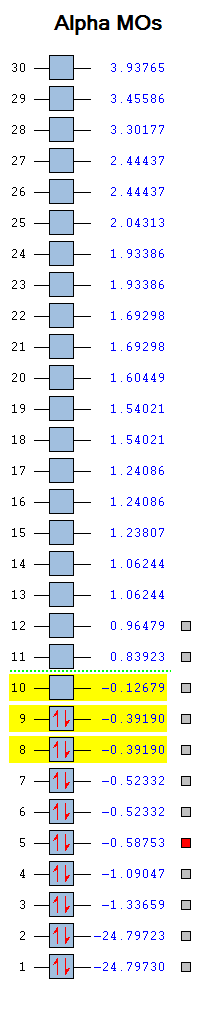
2s Sigma Bonding Molecular Orbital

This is Fluorine's bonding 2s Molecular Orbital which is made up of the Fluorine atoms 2s atomic orbitals combining in phase. It isn't that deep in energy with an energy of -1.33659. It is not the HOMO and does not lie in the HOMO-LUMO region.
2p Sigma Bonding Molecular Orbital

This is Fluorine's bonding 2p Molecular Orbital which is made up of the Fluorine atoms 2p atomic orbitals overlapping head on and combining. It is not very deep in energy with an energy of -0.58753. It is not the HOMO but it does lie near the HOMO-LUMO region.
2p Sigma Anti-Bonding Molecular Orbital

This is Fluorine's empty anti-bonding 2p sigma* Molecular Orbital which is made up of the Fluorine atoms 2p atomic orbitals overlapping out of phase. It is not very deep in energy with an energy of -0.12679. It is the LUMO and it does lie in the HOMO-LUMO region.
2p Pi Bonding Molecular Orbital

This is Fluorine's occupied bonding 2p Pi Molecular Orbital which is made up of the Fluorine atoms 2p atomic orbitals overlapping in phase. It is not deep in energy with an energy of -0.52332. It isn't the HOMO and lies near the HOMO-LUMO region. Fluorine has another occupied 2p pi Molecular Orbital which is degenerate in energy. These are located orthogonal to each other.
2p Pi Anti-Bonding Molecular Orbital

This is Fluorine's occupied anti-bonding 2p Pi* Molecular Orbital which is made up of the Fluorine atoms 2p atomic orbitals overlapping out of phase. It is not deep in energy with an energy of -0.39190. It is the HOMO and lies in the HOMO-LUMO region. Fluorine has another occupied 2p pi* Molecular Orbital which is degenerate in energy. These are located orthogonal to each other. Due to the presence of the filled Pi* Molecular Orbitals, the F2 molecule does not have a Pi bond as they the Pi* cancel out the Pi Molecular Orbitals.
- ↑ Molecular Geometry and Bonding Theories, Molecular Geometry and Bonding Theories, https://www.pearsonhighered.com/blbmw13einfo/assets/pdf/blbmw-ch09.pdf, 350-351
- ↑ Silverstein, R.M.; Bassler, G.C.; and Morrill, T.C. Spectrometric Identification of Organic Compounds. 4th ed. New York: John Wiley and Sons, 1981
