Rep:Mod:al7215
NH3 Molecule
| NH3 Molecule Optimisation | |||
|---|---|---|---|

|
|||
| Information | |||
| Calculation Method | RB3LYP | ||
| Basis Set | 6-31G(d,p) | ||
| E(RB3LYP)/ a.u | -56.55776873 | ||
| RMS Gradient | 0.00000485 | ||
| Point Group | C3V | ||
| Click here for the Log File. | |||
Optimised N-H bond distance for NH3 molecule: 1.01798 Å
Optimised H-N-H bond angle for NH3 molecule: 105.741o
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES
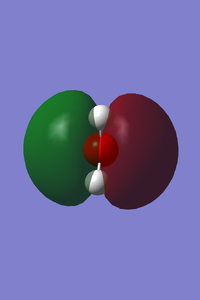
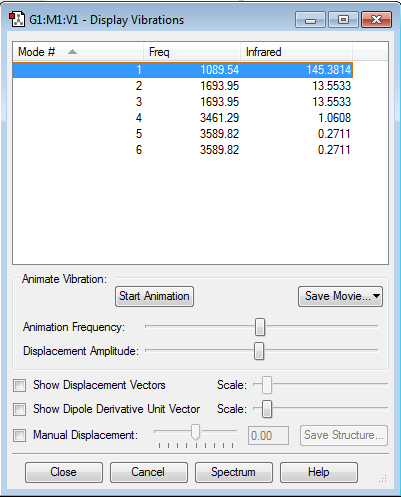
| Vibrational and Charge Analysis of NH3 Molecule | |
|---|---|
|

|
Number of modes expected from 3N-6 ruleː 6
Degenerate modesː Modes 2 and 3 are degenerate; Modes 5 and 6 are degenerate as well.
Bending vibration modesː Modes 1, 2 and 3
Bond stretching vibration modesː Modes 4, 5 and 6
Highly symmetric modeː Mode 4
"Umbrella" modeː Mode 1
Expected number of experimental spectrum bands of gaseous ammoniaː 2 bands, one due to vibration mode 1 and another due to vibration modes 2 and 3. The infrared intensity due to vibration modes 4, 5 and 6 are 1.0608, 0.2711 and 0.2711 respectively. Bands due to these vibration modes are most likely absent from the experimental spectrum as they are extremely low in intensity and can hardly be detected by the instrument.
Expectation of Charge for N and H atoms:The specific Natural Bond Orbital (NBO) charges were analysed for the optimised NH3 molecule. Nitrogen has a negative charge of -1.125 while hydrogen has a positive charge of 0.375. This charge distribution is expected as nitrogen is more electronegative than hydrogen, thus leading to the withdrawal of electrons from the four coordinating hydrogen atoms by nitrogen. Additionally, all four hydrogen atoms have the same charge as all N-H bond lengths are equal.
N2 and H2 Molecules
| N2 Molecule Optimisation | |||
|---|---|---|---|

|
|||
| Information | |||
| Calculation Method | RB3LYP | ||
| Basis Set | 6-31G(d,p) | ||
| E(RB3LYP)/ a.u | -109.52412868 | ||
| RMS Gradient | 0.00000060 | ||
| Point Group | D∞h | ||
| Click here for the Log File. | |||
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
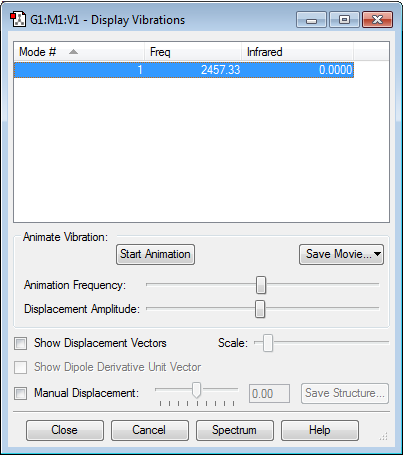
| Vibrational and Charge Analysis of N2 Molecule | |
|---|---|
|
|
There are no negative frequencies. Infrared intensity is zero as there is no change in dipole moment due to symmetric stretching of N2 molecule. Additionally, since there is no electronegativity difference between the two atoms as N2 is a homonuclear diatomic molecule, both atoms will have zero charge.
| H2 Molecule Optimisation | |||
|---|---|---|---|

|
|||
| Information | |||
| Calculation Method | RB3LYP | ||
| Basis Set | 6-31G(d,p) | ||
| E(RB3LYP)/ a.u | -1.17853936 | ||
| RMS Gradient | 0.00000017 | ||
| Point Group | D∞h | ||
| Click here for the Log File. | |||
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES
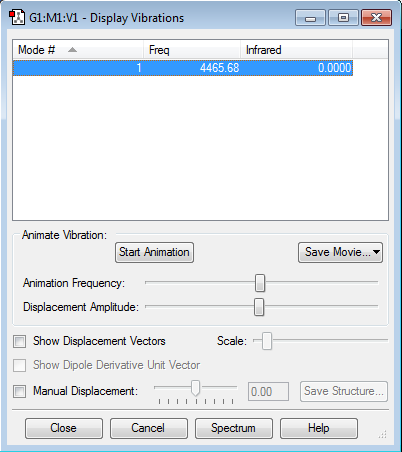
| Vibrational and Charge Analysis of H2 Molecule | |
|---|---|
|
|
Similarly, there are no negative frequencies. Infrared intensity is zero as there is no change in dipole moment due to symmetric stretching of H2 molecule. Additionally, since there is no electronegativity difference between the two atoms as H2 is a homonuclear diatomic molecule, both atoms will have zero charge.
Reaction Energies
Reaction: N2 + 3H2 -> 2NH3
E(NH3) = -56.55776873 a.u.
2*E(NH3) = -113.11553746 a.u.
E(N2) = -109.52412868 a.u.
E(H2) = -1.17853936 a.u.
3*E(H2) = -3.53561808 a.u.
ΔE=2*E(NH3)-[E(N2)+3*E(H2)] = -0.0557907 a.u. = -146.4784829 kJ/mol = -146.48 kJ/mol (2 decimal places)
The energy for converting hydrogen and nitrogen gas into ammonia gas is -146.48 kJ/mol (2 decimal places). The literature value for this Haber process is -109 kJ/mol at 500oC. [1] A possible explanation for the large deviation between the computed and literature value is that the NH3 molecule is set to be under an isolated gas phase for computational analysis, whereas experimentally, NH3 molecule is surrounded by other NH3 molecules. Additionally, temperature also has an effect on how exothermic this reaction would be, which cannot be properly accounted for with computational analysis. This ultimately shows us that simple Density Function Theory (DFT) method is insufficient to help us calculate thermodynamic data.
Since this is an exothermic reaction, the gaseous reactants are at a higher energy level than the ammonia product. Therefore, the ammonia product is more stable than the gaseous reactants.
SiH4 (Silane) Molecule
| SiH4 Molecule Optimisation | |||
|---|---|---|---|

|
|||
| Information | |||
| Calculation Method | RB3LYP | ||
| Basis Set | 6-31G(d,p) | ||
| E(RB3LYP)/ a.u | -291.88802760 | ||
| RMS Gradient | 0.00000002 | ||
| Point Group | Td | ||
| Click here for the Log File. | |||
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
Optimised Si-H bond distance for SiH4 molecule: 1.48485 Å
Optimised H-Si-H bond angle for SiH4 molecule: 109.471o
Frequency and Vibrational modes of SiH4
| No. | Image | Frequency (cm-1) | Infrared | Brief description | ||
| 1 | 919.21 | 136.1485 | Asymmetric Scissoring. Threefold degeneracy due to symmetry of system and three different scissoring motions. As intensity is non-zero, these are infrared active vibration modes due to a change in dipole moment. | |||
| 2 | 919.21 | 136.1485 | ||||
| 3 | 919.21 | 136.1485 | ||||
| 4 | 978.78 | 0.0000 | Symmetric Scissoring. Twofold degeneracy due to symmetry of system and two different scissoring motions. As intensity is zero, these two vibration modes are infrared inactive as there is no change in dipole moment. | |||
| 5 | 978.78 | 0.0000 | ||||
| 6 | 2244.40 | 0.0000 | Symmetric Stretching. As intensity is zero, this vibration mode is infrared inactive as there is no change in dipole moment. | |||
| 7 | 2254.90 | 143.3961 | Asymmetric Stretching. Threefold degeneracy due to symmetry of system and three different ways of stretching. As intensity is non-zero, these are infrared active vibration modes due to a change in dipole moment. | |||
| 8 | 2254.90 | 143.3961 | ||||
| 9 | 2254.90 | 143.3961 |
Charge Analysis for SiH4
Molecular Orbital (MO) Analysis of SiH4
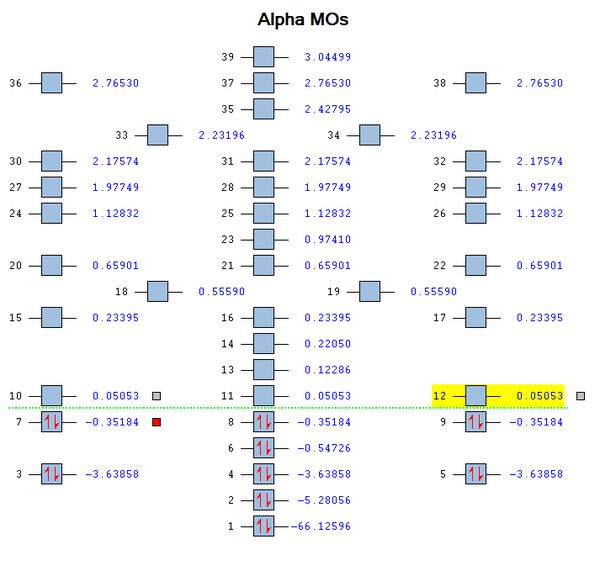
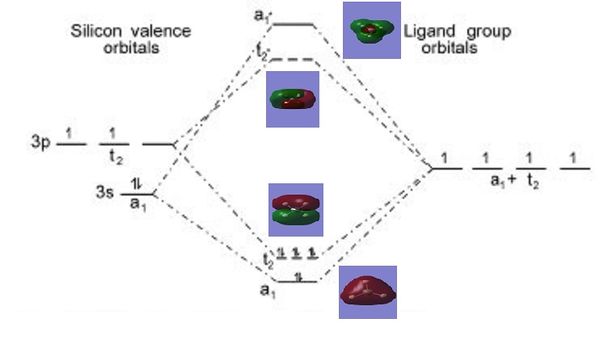
Figure 2 shows the ordering of the various energy levels with their corresponding molecular orbitals generated in gaussview. As a further study, the linear combination of atomic orbitals (LCAO) can also be compared with the computed molecular orbitals to show that the LCAO theory is a fairly good method to predict molecular orbitals for an unknown simple molecule without having to do complicated calculations.
H2O Molecule
| H2O Molecule Optimisation | |||
|---|---|---|---|

|
|||
| Information | |||
| Calculation Method | RB3LYP | ||
| Basis Set | 6-31G(d,p) | ||
| E(RB3LYP)/ a.u | -76.41973740 | ||
| RMS Gradient | 0.00006276 | ||
| Point Group | C2V | ||
| Click here for the Log File. | |||
Item Value Threshold Converged? Maximum Force 0.000099 0.000450 YES RMS Force 0.000081 0.000300 YES Maximum Displacement 0.000114 0.001800 YES RMS Displacement 0.000119 0.001200 YES
Optimised O-H bond distance for H2O molecule: 0.96522 Å
Optimised H-O-H bond angle for H2O molecule: 103.745o
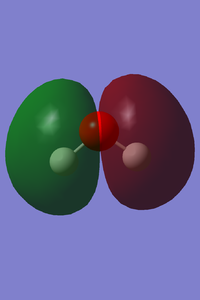
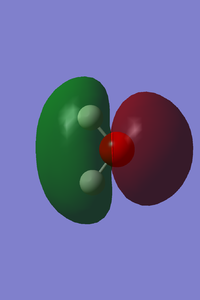
| Vibrational and Charge Analysis of H2O Molecule | |
|---|---|
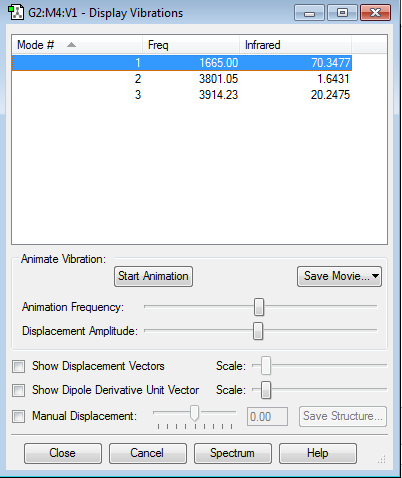
|
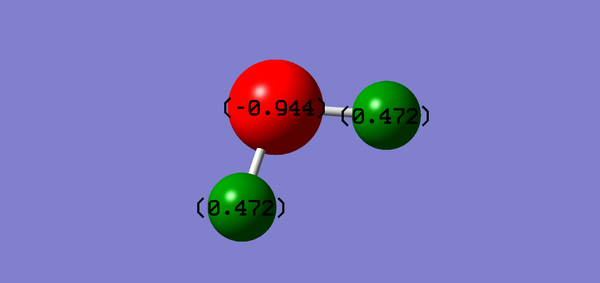
|