Rep:Mod:ak8916
EX3 Section
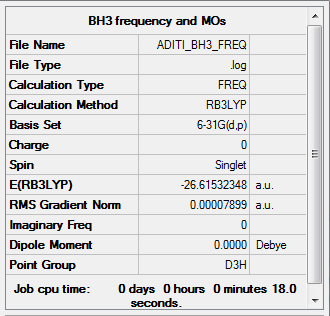
BH3
RB3LYP/6-31G (d.p) level
ie the computational level and basis set
What is the final energy in atomic units (au)? -26.46226371
What is the gradient? 0.00008756
What is the dipole moment? 0.0003
What is the point group of your molecule? D3h
How long did your calculation take? 22 seconds
Item Value Threshold Converged? Maximum Force 0.000217 0.000450 YES RMS Force 0.000105 0.000300 YES Maximum Displacement 0.000692 0.001800 YES RMS Displacement 0.000441 0.001200 YES
Frequency Analysis
Low frequencies --- -0.2456 -0.1129 -0.0055 44.0270 45.1846 45.1853 Low frequencies --- 1163.6049 1213.5924 1213.5951
Optimised BH Molecule |
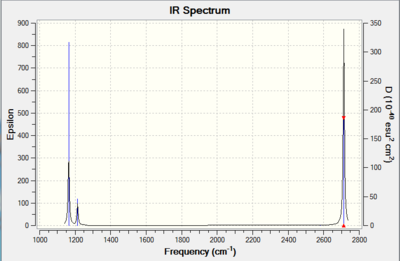
Vibrational spectrum for BH3
| wavenumber (cm-1 | Intensity (arbitrary units) | symmetry | IR active? | type |
| 1164 | 92 | A1' | yes | stretching |
| 1214 | 14 | E' | very slight | bend |
| 1214 | 14 | E' | very slight | bend |
| 2580 | 0 | A1' | no | symmetric stretch |
| 2713 | 126 | E' | yes | asymmetric stretch |
| 2713 | 126 | E' | yes | asymmetric stretch |
Smf115 (talk) 16:32, 28 May 2018 (BST)Good structure information and the molecules is of the correct point group (D3h), however, the symmetries assigned aren't correct and it hasn't been explained why only 3 peaks are visible on the IR spectrum.
MO Diagram Analysis
Looking at the real MOs and comparing them to the expected ones:
1. Are there any significant differences between the real and LCAO MOs?
In general, there are not major differences between the predicted MOs using theory and the real MOs produced by the system.
2. What does this say about the accuracy and usefulness of qualitative MO theory?
To a great extent, the MO theory can be said to be very accurate in determining the expected regions of electron densities, nodes and combination of atomic orbitals.
However, one oddity which props out is that of the third to last orbital where in the predicted MO has a lot of electron density centered on the Boron while the real one has greater electron density at the hydrogen atoms.

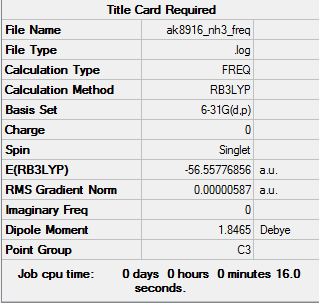
NH3
RB3LYP/6-31G (d.p) level ie the computational level and basis set
The expected point group of this is C3v rather than the one that the summary table depicts above. Constant interpretation of results is essential while using the tool of computational chemistry.
Item Value Threshold Converged?
Maximum Force 0.000013 0.000450 YES
RMS Force 0.000006 0.000300 YES
Maximum Displacement 0.000039 0.001800 YES
RMS Displacement 0.000013 0.001200 YES
Predicted change in Energy=-3.862150D-10
Optimization completed.
-- Stationary point found.
Frequency Analysis
Frequency File: AK8916_NH3_FREQ.LOG
Optimised BH Molecule |
Low frequencies --- -8.5646 -8.5588 -0.0047 0.0454 0.1784 26.4183 Low frequencies --- 1089.7603 1694.1865 1694.1865
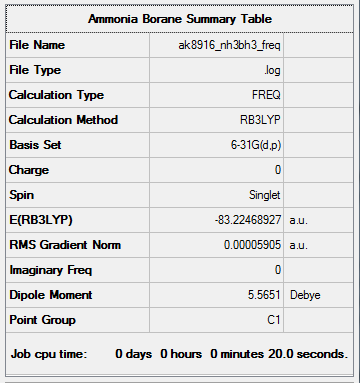
NH3BH3
Item Value Threshold Converged?
Maximum Force 0.000111 0.000450 YES
RMS Force 0.000059 0.000300 YES
Maximum Displacement 0.000663 0.001800 YES
RMS Displacement 0.000385 0.001200 YES
Predicted change in Energy=-1.745833D-07
Optimization completed.
-- Stationary point found.
Frequency Analysis
Frequency File: AK8916_NH3BH3_FREQ.LOG
Low frequencies --- -0.0008 -0.0005 0.0007 13.3858 21.9013 41.2449 Low frequencies --- 266.6364 632.2043 639.1458
Optimised BH Molecule |
Performing Calculations
- E (NH3) = -56.55776856 a.u.
- E (BH3) = -26.61532348 a.u.
- E (NH3)-BH3) = -83.22468927 a.u.
- ΔE=E(NH3)-BH3)- [E(NH3)+E(BH3)
- ΔE=-0.05159723 a.u. = 135.46853768 kj/mol ≃ 135 kJ/mol
The table below gives bond strengths of typical C-C, B-B and N-N bonds and upon comparison it is evident that the dative bond strength observed for NH3BH3 is comparable to weak bonding as the value is lower than all three bonds we are comparing it to.
| Bond | Bond Strength (kJ/mol) |
| C-C | 346 |
| B-B | 293 |
| N-N | 167 |
BBr3
Item Table
Item Value Threshold Converged?
Maximum Force 0.000024 0.000450 YES
RMS Force 0.000014 0.000300 YES
Maximum Displacement 0.000131 0.001800 YES
RMS Displacement 0.000089 0.001200 YES
Predicted change in Energy=-3.306546D-09
Optimization completed.
-- Stationary point found.
Frequency Analysis
Low frequencies --- -3.0398 -0.0002 -0.0001 -0.0001 2.1413 3.6716 Low frequencies --- 155.9081 155.9799 267.7043
DSpace file link: http://hdl.handle.net/10042/202447
Frequency File: BBr3_freq_analysis_pseudo_potentials_final.log
Optimised BH Molecule |
ie your jmol image
Smf115 (talk) 16:31, 28 May 2018 (BST)Correct calculation and good comparisons made. However, the literature values must be referenced and consider the necessary accuracy when reporting the energies of the molecules in a.u. to improve. The accuracy for the final reported dissociation energy is correct though.
Project Section: Lewis Acids and Bases
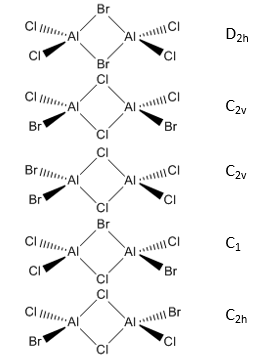
Study Al2Cl4Br2
- 5 different isomers and their point groups
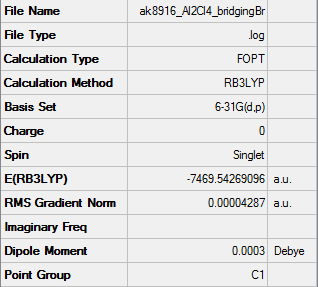
Optimisation of Bridging Bromines
Item Table
Item Value Threshold Converged? Maximum Force 0.000062 0.000450 YES RMS Force 0.000028 0.000300 YES Maximum Displacement 0.000855 0.001800 YES RMS Displacement 0.000525 0.001200 YES
Summary Table
Frequency Analysis
Low frequencies --- -4.7410 -3.9765 -3.6309 -0.0122 0.0051 0.0109 Low frequencies --- 10.4132 65.1068 88.4798
Optimised BH Molecule |
ie your jmol image
Frequency File: AK8916_AL2CL4_BRIDGINGBR_FEQ_OPT.LOG
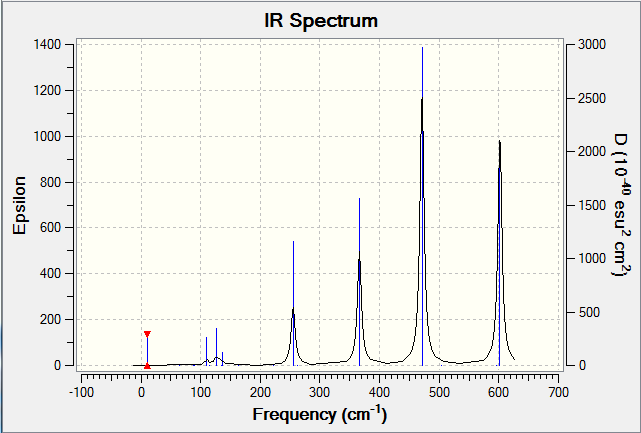
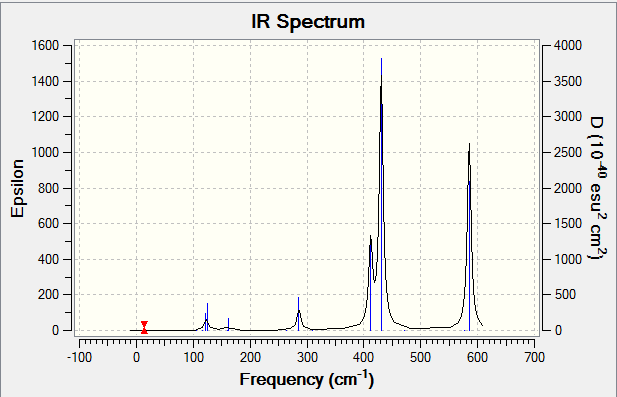
IR Spectrum
Smf115 (talk) 15:40, 1 June 2018 (BST)Good structure information, however, the wrong method has been used for the calculations resulting in incorrect energies. The LanL2DZ psuedo-potential and basis set should have been used for the Br and the 6-31G(dip) basis set for Al and Cl.
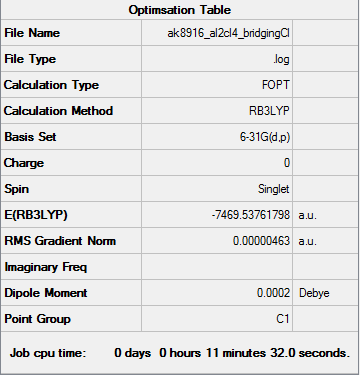
Optimisation of Bridging Cl and Trans Terminal Bromines
Item Table
Item Value Threshold Converged? Maximum Force 0.000007 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000103 0.001800 YES RMS Displacement 0.000050 0.001200 YES
Summary Table
Frequency Analysis
Low frequencies --- -7.4670 -1.7427 -0.0110 -0.0109 0.0107 1.6094 Low frequencies --- 14.4390 51.8515 73.7896
Optimised BH Molecule |
ie your jmol image
Frequency File: Media: AK8916_AL2CL4_BRIDGINGCL_FREQ_OPT.LOG
IR Spectrum
Comparing the two isomers
Energy of bridging Chlorines isomer is lower than that of the energy of bridging Bromines and is therefore the more stable conformer.
- E(Bridging Br conformer) = -7469.54269096 a.u.
- E(Bridging Cl and trans Br)= -7469.53761798 a.u.
- Relative Energy= 0.00507298 a.u. = 13.319110005 ≃ 13 kJ/mol
Where the bridging Br isomer is the more stable one. This can be explained theoretically as bridging bromine would be a better sigma donor to the electron deficient metal due its lower electronegativity compared to chlorine.
However, we may expect Chlorine orbitals to have better orbital overlap as both Cl and Al are in the same period. Due to these two opposing factors, the computed energy difference has a small value of 13 kJ/mol.
Computation here concludes that electronegatvity factor over-rides the orbital overlap argument in this molecule and the more stable energy isomer is the one with bridging bromines.
Computing energy of AlCl2Br
Optimisation of Molecule
Items Table
Item Value Threshold Converged? Maximum Force 0.000021 0.000450 YES RMS Force 0.000014 0.000300 YES Maximum Displacement 0.000164 0.001800 YES RMS Displacement 0.000105 0.001200 YES
Summary Table
Frequency Analysis
Low frequencies --- -2.8061 -2.1897 0.0062 0.0130 0.0140 5.8239 Low frequencies --- 125.0193 137.5089 194.8968
Optimised BH Molecule |
ie your jmol image
Frequency File: AK8916_ALCL2BR_FREQ_OPT.LOG
Dissociation energy from lowest energy conformer into 2AlCl2Br
Is product more stable than individual monomers?
Product is more stable than the monomers because E(2 AlCl2Br) > E(Al2Cl4Br2)
The energy difference between (2 AlCl2Br) and the most stable isomer of Al2Cl4Br2 is 0.04565132 a.u. which is 119.85754979 kJ/mol or approximately 120 kJ/mol
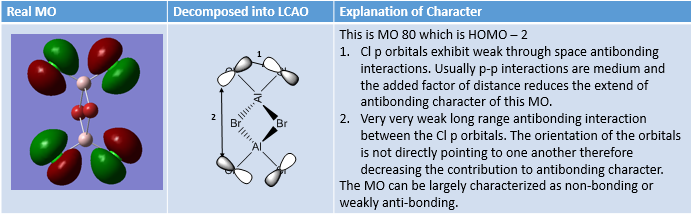
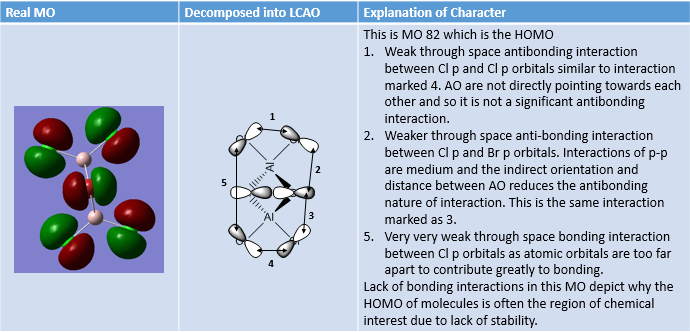
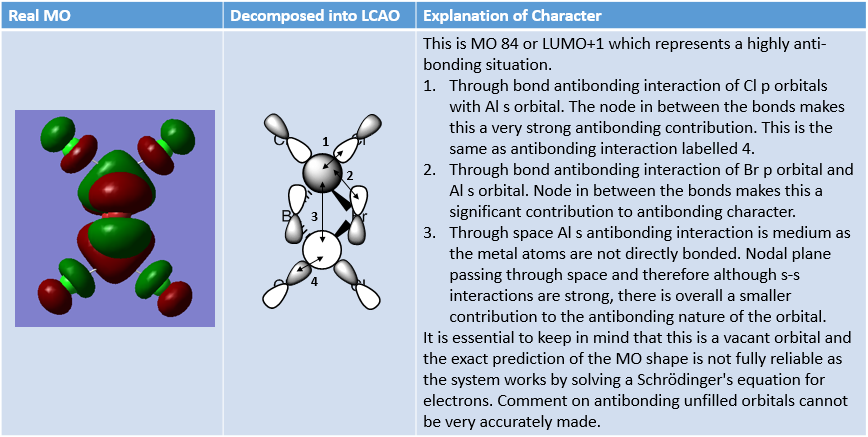
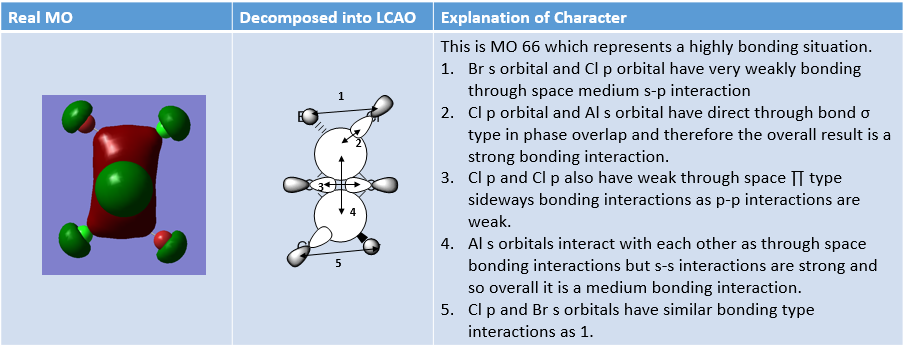
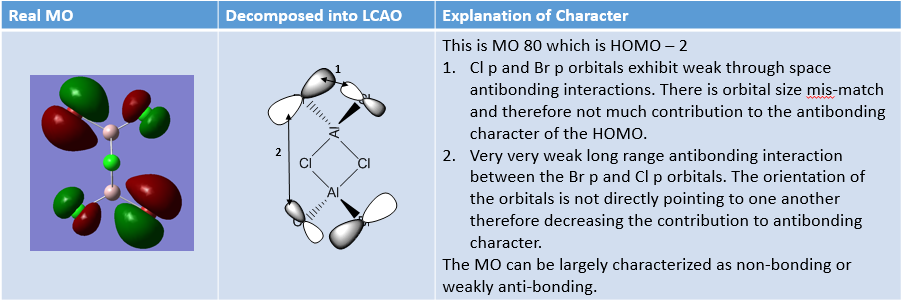
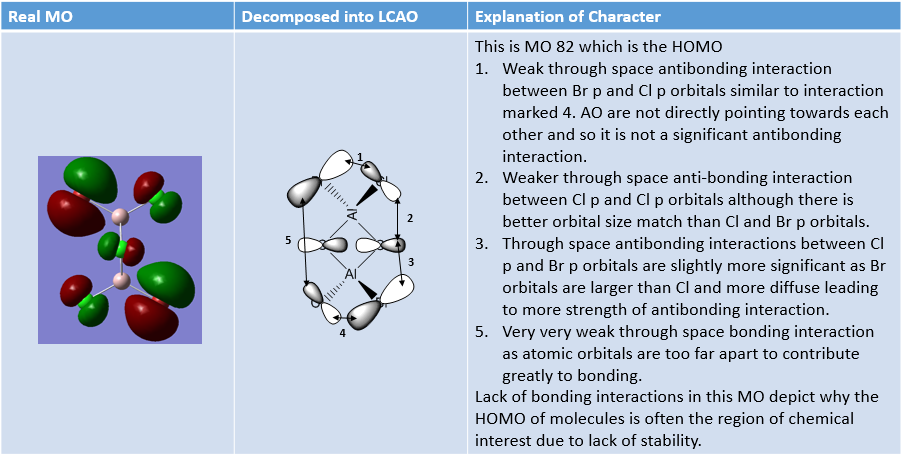
MO Analysis of same MOs of the Higher and Lower Energy Isomer
The analysis carried out below outlines interesting features about the two isomers.
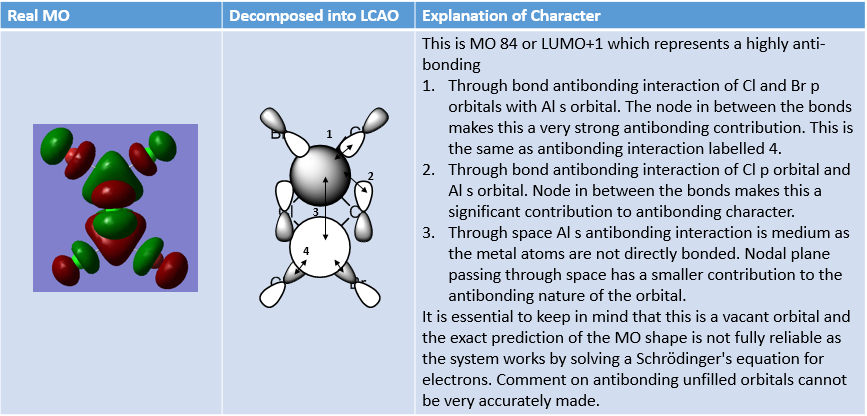
MO Analysis of 4 orbitals of Lowest Energy Isomer
The figures below depict movement from highly bonding to highly antibonding MOs.
MO Analysis of 4 Orbitals of Higher Energy Isomer
Smf115 (talk) 16:00, 1 June 2018 (BST)Thorough analysis of the main interactions and correct LCAOs for the MOs chosen for both isomers. Nice to see the additional analysis for the second isomer and while some comparisons were made it would have been good to see some more (but extra information!). The identification of the bonding character is good however, the annotations aren't the clearest and are hard to follow. Additional details such as labelling the nodal planes would also improve the analysis.
Smf115 (talk) 16:00, 1 June 2018 (BST)Overall, a good wiki report and good attempt at the project section.