Rep:Mod:ag01500903
Molecular Modelling
NH3
File:01500903ANNAGUO NH3OPT.LOG
OPT information
| calculation method | basis set | final energy | RMS gradient | point group |
|---|---|---|---|---|
| RB3LYP | 6-31G(d.p.) | -56.55776873a.u. | 0.00000485a.u. | C3V |
N-H bond distance= 1.01798Å (accurate to ≈ 0.01Å)
H-N-H bond angle= 105.741° (accurate to ≈ 1°)
real output--item table
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES
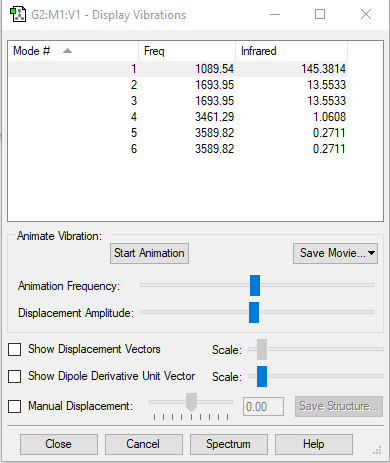
vibration
| wavenumber cm-1 | 1090 | 1694 | 1694 | 3461 | 3590 | 3590 |
| symmetry | A1 | E | E | A1 | E | E |
| intensity arbitrary units | 145 | 14 | 14 | 1 | 0 | 0 |
| image |  |
 |
 |
 |
 |
|
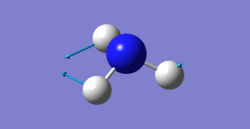
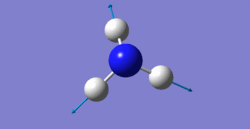
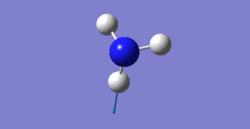
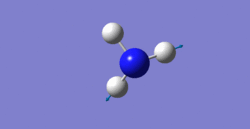
6 modes are expected from the 3N-6 rule.
The second and third modes are degenerated which have wavenumber of 1694cm-1.
Also, the fifth and sixth modes are degenerated which have wavenumber of 3590cm-1.
bending: 1090cm-1, 1694cm-1, 1694cm-1
stretching: 3461cm-1, 3590cm-1, 3590cm-1
3461cm-1 one is highly symmetric.
1090cm-1 one is umbrella mode.
2 bands are expected in experimental spectrum.
charge distribution
charge on N = -1.125
charge on H = +0.375
N is expected to be negatively charged and H is expected to be positively charged because N is more electronegative.
N2
OPT information
| calculation method | basis set | final energy | RMS gradient | point group |
|---|---|---|---|---|
| RB3LYP | 6-31G(d.p.) | -109.52412868a.u. | 0.00000134a.u. | D∞h |
N-N triple bond distance= 1.1055Å (accurate to ≈ 0.01Å)
real output--item table
Item Value Threshold Converged? Maximum Force 0.000002 0.000450 YES RMS Force 0.000002 0.000300 YES Maximum Displacement 0.000001 0.001800 YES RMS Displacement 0.000001 0.001200 YES
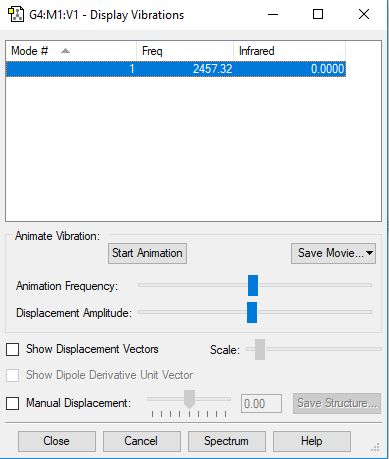
vibration
| wavenumber cm-1 | 2457 |
| symmetry | SGG |
| intensity arbitrary units | 0 |
| image | 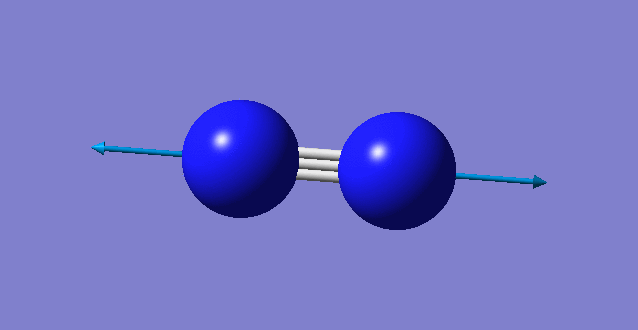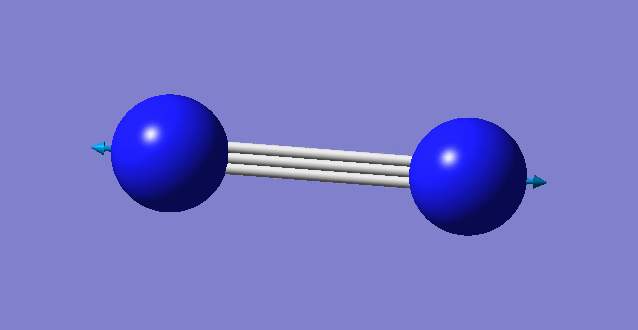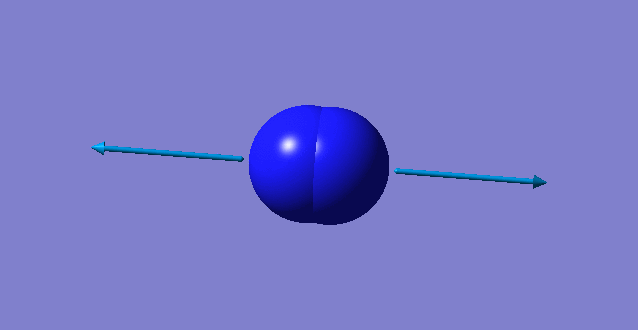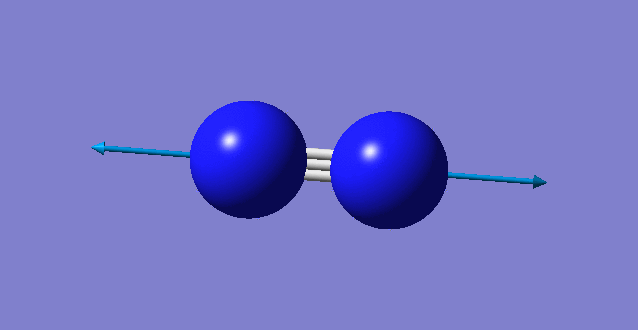
|
charge distribution
H2
OPT information
| calculation method | basis set | final energy | RMS gradient | point group |
|---|---|---|---|---|
| RB3LYP | 6-31G(d.p.) | -1.17853936a.u. | 0.00000023a.u. | D∞h |
H-H bond distance= 0.74279Å (accurate to ≈ 0.01Å)
real output--item table
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000001 0.001800 YES RMS Displacement 0.000001 0.001200 YES
vibration
| wavenumber cm-1 | 4466 |
| symmetry | SGG |
| intensity arbitrary units | 0 |
| image | 
|
charge distribution
Cl2
OPT information
| calculation method | basis set | final energy | RMS gradient | point group |
|---|---|---|---|---|
| RB3LYP | 6-31G(d.p.) | -920.3498788a.u. | 0.00008611a.u. | D∞h |
Cl-Cl bond distance= 2.04117Å (accurate to ≈ 0.01Å)
real output--item table
Item Value Threshold Converged? Maximum Force 0.000149 0.000450 YES RMS Force 0.000149 0.000300 YES Maximum Displacement 0.000415 0.001800 YES RMS Displacement 0.000587 0.001200 YES
vibration
| wavenumber cm-1 | 521 |
| symmetry | SGG |
| intensity arbitrary units | 0 |
| image | 
|
charge distribution
complex containing N2
name:tris(m-N-(3,5-dimethylphenyl)di(propan-2-yl)phosphanaminido)-(bis(4-methoxyphenyl)methanone)-dinitrogen-titanium-cobalt diethyl ether solvate
N-N triple bond distance= 1.0773Å
The bond length between N-N triple bond in complex is smaller than that in nitrogen gas. This may because Co next to N donate electron to N, which results in the increase in electronegativity of that N atom, therefore the distance between two nitrogen atoms decreases.
energy for forming NH3
E(NH3)=-56.55776873 a.u.
2*E(NH3)=-113.11553746 a.u.
E(N2)=-109.52412868 a.u.
E(H2)=-1.17853936 a.u.
3*E(H2)=-3.53561808 a.u.
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]=-0.0557907 a.u. = -146.5 kjmol-1
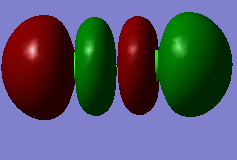
MO for Cl2
This molecular orbital is the sigma bonding between two 1s orbitals of two chlorine atoms, which is deep in energy (-101.60295 a.u.) and occupied by two electrons.
This molecular orbital is the sigma antibonding between two 1s orbitals of two chlorine atoms, which is occupied by two electrons. This orbital is not deep in energy(-9.51828 a.u.).
This molecular orbital is the sigma bonding between two 2p orbitals of two chlorine atoms, which is occupied by two electrons. This orbital is not deep in energy(-7.2859 a.u.).
This molecular orbital is the pi antibonding between two 4p orbitals of two chlorine atoms, which is HOMO of Cl2 and occupied by two electrons. This orbital is not deep in energy(-0.31356 a.u.).
This molecular orbital is the sigma antibonding between two 4p orbitals of two chlorine atoms, which is LUMO of Cl2 and not occupied by electrons. This orbital is not deep in energy(-0.14189 a.u.).
Marking
Note: All grades and comments are provisional and subject to change until your grades are officially returned via blackboard. Please do not contact anyone about anything to do with the marking of this lab until you have recieved your grade from blackboard.
Wiki structure and presentation 1/1
Is your wiki page clear and easy to follow, with consistent formatting?
YES
Do you effectively use tables, figures and subheadings to communicate your work?
YES - well structured wiki good job.
NH3 1/1
Have you completed the calculation and given a link to the file?
YES
Have you included summary and item tables in your wiki?
YES
Have you included a 3d jmol file or an image of the finished structure?
YES
Have you included the bond lengths and angles asked for?
YES
Have you included the “display vibrations” table?
YES
Have you added a table to your wiki listing the wavenumber and intensity of each vibration?
YES
Did you do the optional extra of adding images of the vibrations?
YES
Have you included answers to the questions about vibrations and charges in the lab script?
YES
N2 and H2 0.5/0.5
Have you completed the calculations and included all relevant information? (summary, item table, structural information, jmol image, vibrations and charges)
YES
Crystal structure comparison 0.5/0.5
Have you included a link to a structure from the CCDC that includes a coordinated N2 or H2 molecule?
YES
Have you compared your optimised bond distance to the crystal structure bond distance?
YES - your reasoning is quite difficult to understand however you have recognised the discrepancy due to bonding in the complex.
Haber-Bosch reaction energy calculation 0.5/1
Have you correctly calculated the energies asked for? ΔE=2*E(NH3)-[E(N2)+3*E(H2)]
YES
Have you reported your answers to the correct number of decimal places?
YES
Do your energies have the correct +/- sign?
YES
Have you answered the question, Identify which is more stable the gaseous reactants or the ammonia product?
You have not answered the question.
Your choice of small molecule 2.5/5
Have you completed the calculation and included all relevant information?
YES
Have you added information about MOs and charges on atoms?
You included the charges but no explanation of why they are 0 on both atoms. Some of the MO analysis is good however you have misidentified several of the contributing AOs. The second MO is 2s not 1s and the 4th and 5th MOs are 3p not 4p AOs.
Independence 0/1
If you have finished everything else and have spare time in the lab you could: Check one of your results against the literature, or Do an extra calculation on another small molecule, or Do some deeper analysis on your results so far
No independent work.