Rep:Mod:adb3418
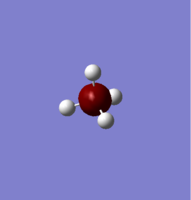
NH3
N-H bond length=1.02 A
H-N-H angle=1060
| Item | Value | Threshold | Converged? |
|---|---|---|---|
| Maximum Force | 0.000004 | 0.000450 | YES |
| RMS Force | 0.000004 | 0.000300 | YES |
| Maximum Displacement | 0.000072 | 0.001800 | YES |
| RMS Displacement | 0.000035 | 0.001200 | YES |
Calculation method
RB3LYP
Basis set
6-31G(d,p)
Final energy E(RB3LYP)
-56.5577687 a.u.
Dipole moment
1.85 Debye
Point group
C3V
JSmol
test molecule |
Display options
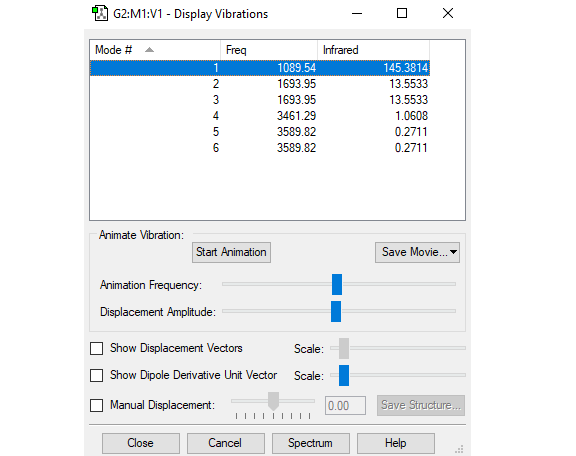
Vibration analysis
| Mode number | 1 | 2 | 3 | 4 | 5 | 6 |
| Wavenumber ( cm-1 ) | 1090 | 1694 | 1694 | 3461 | 3590 | 3590 |
| Symmetry | A1 | E | E | A1 | E | E |
| Intensity (arbitrary units) | 145 | 14 | 14 | 1 | 0 | 0 |
| Image |  |
 |
 |
 |
 |

|
Answered questions
Applying the 3N-6 rule, I would expect 6 vibration modes. The modes that are degenerate are modes 2 (1694 cm-1) and 3 (1694 cm-1), as well as modes 5 (3590 cm-1) and 6 (3590 cm-1). The bond stretch vibration modes are 4,5 and 6. The bending vibration modes are 1,2 and 3. The mode that is highly symmetrical is mode 4. The umbrella mode is mode 1 at 1090 cm-1. I would expect to see only 2 bands in the IR spectrum of ammonia due to the fact that there are degenerate vibration modes and some of the have very low intensity due to very small change in dipole moment. I would expect to see bands at 1090 and 1694 cm-1.
Charge analysis
I would expect a partial positive charge on hydrogen atoms and a partial negative charge on nitrogen based on the fact that nitrogen is more electronegative than hydrogen. The results yielded by Gaussian are consistent with my predictions:
charge on H = +0.375 charge on N = -1.125
LOG file
Media:NH3_OPTIMISATION_ADB3418.LOG
N2
N-N bond length=1.11 A
| Item | Value | Threshold | Converged? |
|---|---|---|---|
| Maximum Force | 0.000001 | 0.000450 | YES |
| RMS Force | 0.000001 | 0.000300 | YES |
| Maximum Displacement | 0.000000 | 0.001800 | YES |
| RMS Displacement | 0.000000 | 0.001200 | YES |
Calculation type
RB3LYP
Basis set
6-31G(d,p)
Final energy E(RB3LYP)
-109.5241287 a.u.
Dipole moment
0.00 Debye
Point group
D∞h
JSmol
test molecule |
Display options
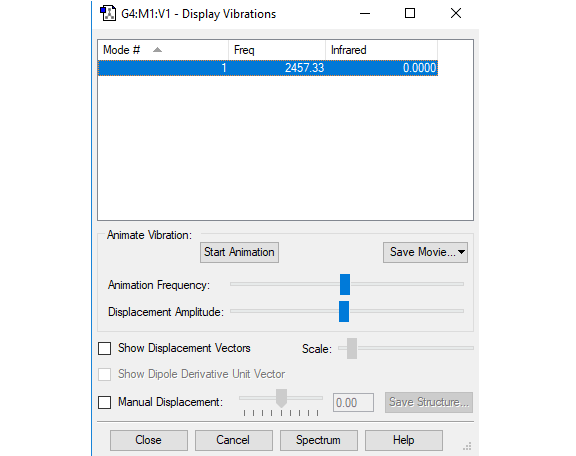
Vibration analysis
| Frequency (cm-1) | 2457 |
| Symmetry | SGG |
| Intensity | 0 |
| Image | 
|
The vibration is not visible in IR because it induces no change in dipole.
Charge analysis
Because it is a homonuclear diatomic molecule, N2 does not exhibit partial charges on the constituent atoms. Electron density is equally shared.
LOG file
H2
H-H bond length=0.74 A
| Item | Value | Threshold | Converged? |
|---|---|---|---|
| Maximum Force | 0.000000 | 0.000450 | YES |
| RMS Force | 0.000000 | 0.000300 | YES |
| Maximum Displacement | 0.000000 | 0.001800 | YES |
| RMS Displacement | 0.000001 | 0.001200 | YES |
Calculation type
RB3LYP
Basis set
6-31G(d,p)
Final energy E(RB3LYP)
-1.1785394 a.u.
Dipole moment
0.00 Debye
Point group
D∞h
JSmol
test molecule |
Display options
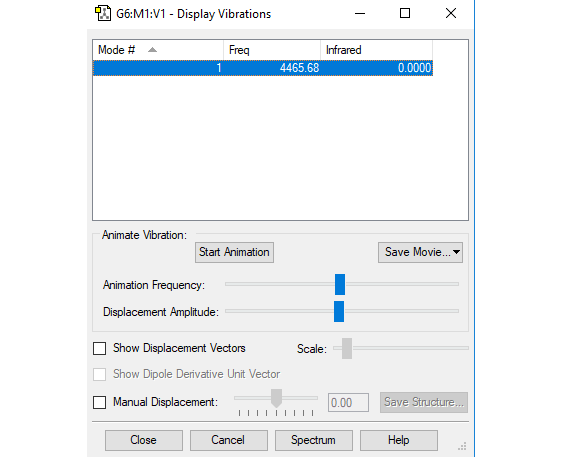
Vibration analysis
| Frequency (cm-1) | 4466 |
| Symmetry | SGG |
| Intensity | 0 |
| Image | 
|
The vibration is not visible in IR because it induces no change in dipole.
Charge analysis
Because it is a homonuclear diatomic molecule, H2 does not exhibit partial charges on the constituent atoms. Electron density is equally shared.
LOG file
H2 in a mono-metallic transition metal complex
The compound that I selected is HINBOV, trichloro-bis(cyclohexylisocyanido)-bis(dimethylphenylphosphine)-rhenium(III) 1,2-dichlorobenzene solvate which coordinates H2. The H-H bond is 0.84 A, which is longer than the calculated H-H bond ( 0.74 A ) in gaseous hydrogen molecule. This deviation is due to the fact that Ru draws electron density from H-H bond in the Ruthenium complex, weakening it and thus making it longer. The values may also be different due to computational errors in our method. To reduce the errors, we could use a more accurate method. [1]
Haber-Bosch process
| E(NH3)=-56.5577687 | a.u. |
| 2*E(NH3)=-113.115537 | a.u. |
| E(N2)=-109.5241287 | a.u. |
| E(H2)=-1.1785394 | a.u. |
| 3*E(H2)=-3.5356182 | a.u. |
ΔE=2*E(NH3)-[E(N2)+3*E(H2)] ΔE=-146.5 kJ/mol
From calculation consideration, the stablest molecule is N2, followed by NH3 and the least stable molecule is H2. Therefore, the gaseous reactants are stabler than the product. Also, according to these calculations, Haber-Bosch process is exothermic, thus the formation of ammonia is favoured.
CH4
C-H bond=1.09 A
H-C-H angle=1100
| Item | Value | Threshold | Converged? |
|---|---|---|---|
| Maximum Force | 0.000063 | 0.000450 | YES |
| RMS Force | 0.000034 | 0.000300 | YES |
| Maximum Displacement | 0.000179 | 0.001800 | YES |
| RMS Displacement | 0.000095 | 0.001200 | YES |
Calculation method
RB3LYP
Basis set
6-31G(d,p)
Final energy E(RB3LYP)
-40.5240140 a.u.
Dipole moment
0.00 Debye
Point group
Td
JSmol
test molecule |
Display options
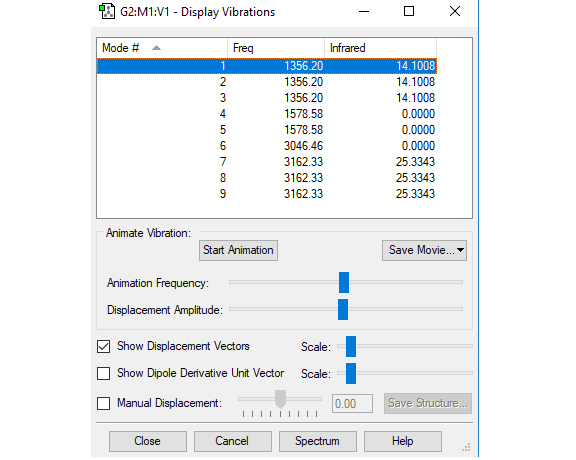
Vibration analysis
| Mode number | 1 | 2 | 3 | 4 |
| Wavenumber ( cm-1 ) | 1356 | 1356 | 1356 | 1579 |
| Symmetry | T2 | T2 | T2 | E |
| Intensity (arbitrary units) | 14 | 14 | 14 | 0 |
| Image |  |
 |
 |

|
| Mode number | 5 | 6 | 7 | 8 | 9 |
| Wavenumber ( cm-1 ) | 1579 | 3046 | 3162 | 3162 | 3162 |
| Symmetry | E | E | A1 | T2 | T2 |
| Intensity (arbitrary units) | 0 | 0 | 0 | 25 | 25 |
| Image |  |
 |
 |
 |

|
Charge analysis
Because it is a non-polar molecule by virtue of the connectivity of the atoms and the very small difference in electronegativities, methane does not present a dipole moment.
Molecular orbitals of CH4
LOG file
Investigation of a reaction
In the process of oil drilling, methane gas is released from pockets of natural gas. Because it is a greenhouse gas that is 86 times more powerful than CO2 over the course of 20 years, it is usually burned in a process called "flaring"[1]. Methane is a very abundant and cheap source of fuel. It is of great interest to find an efficient method of converting methane into a liquid fuel like methanol. Current protocols of oxidising methane to methanol use high temperatures, O2 and nitrogen oxides as catalysts[2]. It is also elusive to prevent further oxidation to CO2. In this section, I want to explore a reaction that has been done in literature before[3] and that can generate methanol from methane. The reaction can be summarised as follows:
CH4 + H2O2 ---> CH3OH + H2O
We can apply the same method as the one used for the Haber-Bosch process. We build each molecule in Gaussian, optimise it and extract the energy parameter. Thus, to estimate the ΔE for this reaction, we deduce the formula:
ΔE=E(CH3OH)+E(H2O)-E(CH4)-E(H2O2)
The values calculated by Gaussian are the following:
| E(CH3OH)=-115.7239644 | a.u. |
| E(H2O)=-76.4197374 | a.u. |
| E(CH4)=-40.5240140 | a.u. |
| E(H2O2)=-151.5431915 | a.u. |
Thus, plugging the numbers in (and converting to kJ/mol) gives a ΔE of -200.8 kJ/mol. According to these calculations, oxidation of methane by hydrogen peroxide is an exothermic reaction. This means that, enthalpically (energetically), this reaction is favourable. Using enthalpies of formation from literature[4], we can obtain a literature value of ΔH for this reaction. Thus, ΔH was calculated to be -261.6 kJ/mol. The difference in values might arise from the fact that the calculation method used assumes this reaction happens in vacuum (it assumes the components are in vacuum). Also, this method is not very accurate and other methods could be used to obtain better results. However, the calculated energy is only part of the picture. Such a reaction cannot happen in the absence of a catalyst. Entropy should also be considered when assessing how favourable a chemical reaction is.
LOG files
| Methanol | Media:METHANOLTRY_adb3418.LOG |
| Hydrogen peroxide | Media:H2O2TRY_adb3418.LOG |
| Water | Media:H2O_adb3418.LOG |
| Methane | Media:METHANE_ADB3418.LOG |
References
Marking
Note: All grades and comments are provisional and subject to change until your grades are officially returned via blackboard. Please do not contact anyone about anything to do with the marking of this lab until you have received your grade from blackboard.
Wiki structure and presentation 0.5/1
Is your wiki page clear and easy to follow, with consistent formatting?
YES
Do you effectively use tables, figures and subheadings to communicate your work?
YES, overall a good structure, however you have used a few too many subheadings which really break up the page and make it hard work for the reader to see the data in context. Also you have left all your jmol captions the same - test molecule - this isn’t a good caption and doesn’t help the reader understand the figure.
NH3 1/1
Have you completed the calculation and given a link to the file?
YES
Have you included summary and item tables in your wiki?
YES
Have you included a 3d jmol file or an image of the finished structure?
YES
Have you included the bond lengths and angles asked for?
YES
Have you included the “display vibrations” table?
YES
Have you added a table to your wiki listing the wavenumber and intensity of each vibration?
YES
Did you do the optional extra of adding images of the vibrations?
YES
Have you included answers to the questions about vibrations and charges in the lab script?
YES, well done for mentioning dipole moment.
N2 and H2 0.5/0.5
Have you completed the calculations and included all relevant information? (summary, item table, structural information, jmol image, vibrations and charges)
YES
Crystal structure comparison 0.5/0.5
Have you included a link to a structure from the CCDC that includes a coordinated N2 or H2 molecule?
YES
Have you compared your optimised bond distance to the crystal structure bond distance?
YES
Haber-Bosch reaction energy calculation 1/1
Have you correctly calculated the energies asked for? ΔE=2*E(NH3)-[E(N2)+3*E(H2)]
YES
Have you reported your answers to the correct number of decimal places?
YES
Do your energies have the correct +/- sign?
YES
Have you answered the question, Identify which is more stable the gaseous reactants or the ammonia product?
YES
Your choice of small molecule 4/5
Have you completed the calculation and included all relevant information?
YES
Have you added information about MOs and charges on atoms?
YES, overall accurate and succinct description, well done! One part which isn't quite right is that MOs 4 and 5 do not contribute to the overall bonding of the molecule - this is because they are unoccupied so have no impact on the positions of electrons in the molecule.
Independence 1/1
If you have finished everything else and have spare time in the lab you could:
Check one of your results against the literature, or
Do an extra calculation on another small molecule, or
Do some deeper analysis on your results so far
You wrote an extra minireport on generating methanol from methane, you used the literature and did extra calculations and analysis, well done this section was really good!
- ↑ https://www.sierraclub.org/sierra/green-life/let-it-burn-congress-allows-flaring-venting-methane-gas
- ↑ https://www.sierraclub.org/sierra/green-life/let-it-burn-congress-allows-flaring-venting-methane-gas
- ↑ Peipei Xiao et al. “Selective oxidation of methane to methanol with H2O2 over an Fe-MFI zeolite catalystusing sulfolane solvent”. In:Chem. Commun.55 (20 2019), pp. 2896–2899.doi:10.1039/C8CC10026H.url:http://dx.doi.org/10.1039/C8CC10026H.4
- ↑ https://www.thoughtco.com/common-compound-heat-of-formation-table-609253