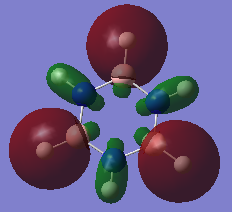Rep:Mod: Brendan Rogers Wiki
BH3
RB3LYP/6-31G(d,p)

Item Value Threshold Converged? Maximum Force 0.000190 0.000450 YES RMS Force 0.000095 0.000300 YES Maximum Displacement 0.000747 0.001800 YES RMS Displacement 0.000374 0.001200 YES
Frequency analysis log file: Media:BR BHS FREQ.LOG
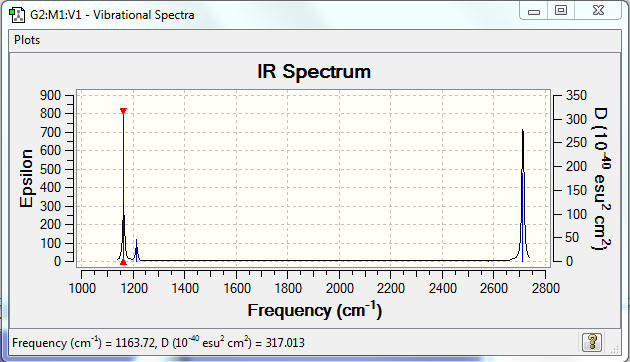
Low frequencies --- -0.2260 -0.1035 -0.0054 48.0278 49.0875 49.0880 Low frequencies --- 1163.7224 1213.6715 1213.6741
BH |
Vibrational spectrum for BH3
| wavenumber (cm-1 | Intensity (arbitrary units) | symmetry | IR active? | type |
| 1164 | 92 | A | yes | out-of-plane-bend |
| 1214 | 14 | E' | very slight | bend |
| 1214 | 14 | E' | very slight | bend |
| 2580 | 0 | A' | no | symmetric stretch |
| 2713 | 126 | E' | yes | asymmetric stretch |
| 2713 | 126 | E' | yes | asymmetric stretch |
BH3 MO diagram
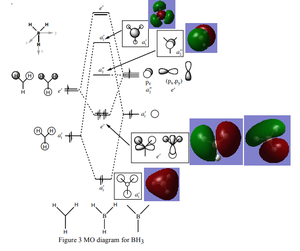
For all occupied orbitals the AO combinations produced fairly accurate results. They also gave fairly accurate results for the a2 unoccupied MO, but not for a1' MO diagram. This shows that using linear combinations of AOs can produce good estimations, but can be inaccurate in reality.
Ng611 (talk) 15:45, 21 May 2018 (BST) I'd say the qualitative MO for a1' is fairly accurate.
Ng611 (talk) 15:44, 21 May 2018 (BST) Where are your orbitals for e'?
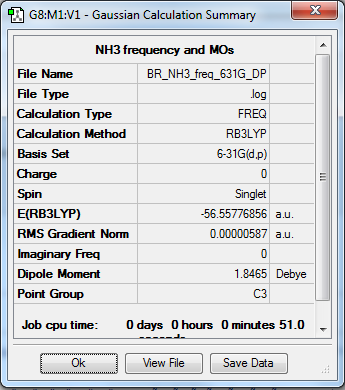
NH3
B3LYP/6-31G(d,p)
Item Value Threshold Converged? Maximum Force 0.000013 0.000450 YES RMS Force 0.000006 0.000300 YES Maximum Displacement 0.000039 0.001800 YES RMS Displacement 0.000013 0.001200 YES
Frequency file: File:BR NH3 FREQ 631G DP.LOG
Low frequencies --- -8.5646 -8.5588 -0.0041 0.0455 0.1784 26.4183 Low frequencies --- 1089.7603 1694.1865 1694.1865
NH |
NH3BH3
B3LYP/6-31G(d,p)
Item Value Threshold Converged? Maximum Force 0.000115 0.000450 YES RMS Force 0.000060 0.000300 YES Maximum Displacement 0.000584 0.001800 YES RMS Displacement 0.000345 0.001200 YES
Frequency file: File:BR NH3BH3 FREQ 631G DP 2.LOG
Low frequencies --- 0.0004 0.0009 0.0012 16.4186 17.2680 37.2272 Low frequencies --- 265.8841 632.2034 639.2870
NHBr |
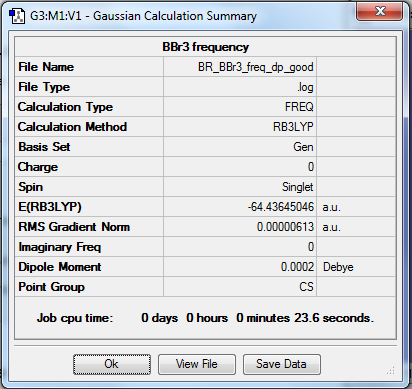
BBr3
B3LYP/6-31G(d,p)
Item Value Threshold Converged? Maximum Force 0.000013 0.000450 YES RMS Force 0.000006 0.000300 YES Maximum Displacement 0.000058 0.001800 YES RMS Displacement 0.000034 0.001200 YES
Frequency file: File:BR BBr3 freq dp good.log
Low frequencies --- -1.9018 -0.0001 0.0002 0.0002 1.5796 3.2831 Low frequencies --- 155.9053 155.9625 267.7047
BBr |
Calculating Association energy of Borane
E(NH3)= -56.55777 a.u. E(BH3)= -26.61532 a.u. E(NH3BH3)= -83.22469 a.u.
ΔE=E(NH3BH3)-[E(NH3)+E(BH3)] = -83.22469 - [-56.55777 + -26.61532] = -0.05160 a.u. = -129 Kj/mol
Ng611 (talk) 15:49, 21 May 2018 (BST) You got the correct a.u. value, but you've converted to kj/mol incorrectly!
This value shows it is a weak bond, as can be shown by comparing it to a strong bond (e.g. C-C bond strength is 346 Kj/mol) and seeing the value is far lower.
Ng611 (talk) 15:49, 21 May 2018 (BST) Remember to cite your bond values (ideally from a textbook, databook, or paper).
Aromaticity
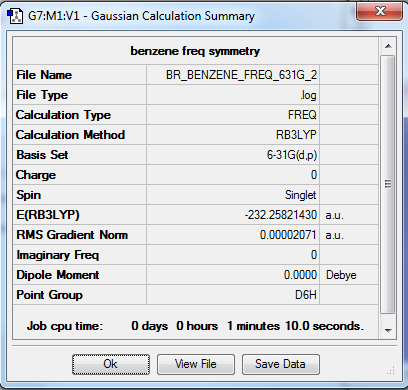
Benzene
B3LYP/6-31G(d,p)
Item Value Threshold Converged? Maximum Force 0.000101 0.000450 YES RMS Force 0.000033 0.000300 YES Maximum Displacement 0.000158 0.001800 YES RMS Displacement 0.000068 0.001200 YES
Frequency file: File:BR BENZENE FREQ 631G 2.LOG
Low frequencies --- -17.1502 -13.8161 -0.0003 -0.0002 -0.0002 1.7484 Low frequencies --- 414.1959 414.6468 620.9365
Benzene |
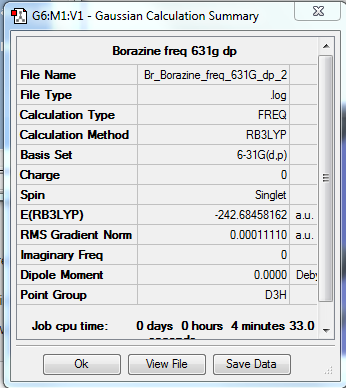
Borazine
B3LYP/6-31G(d,p)
Item Value Threshold Converged? Maximum Force 0.000268 0.000450 YES RMS Force 0.000111 0.000300 YES Maximum Displacement 0.000537 0.001800 YES RMS Displacement 0.000253 0.001200 YES
Frequency file: File:BR BORAZINE FREQ 631G DP 2.LOG
Low frequencies --- -14.1757 -13.8386 -10.2802 -0.0163 -0.0114 0.0344 Low frequencies --- 289.0978 289.0995 404.2449
Borazine |
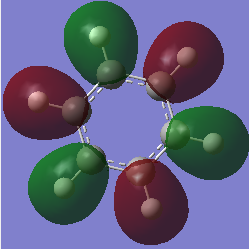
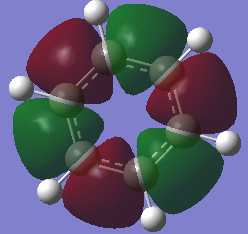
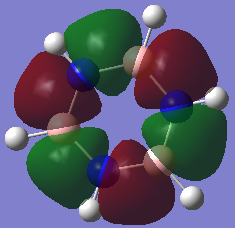
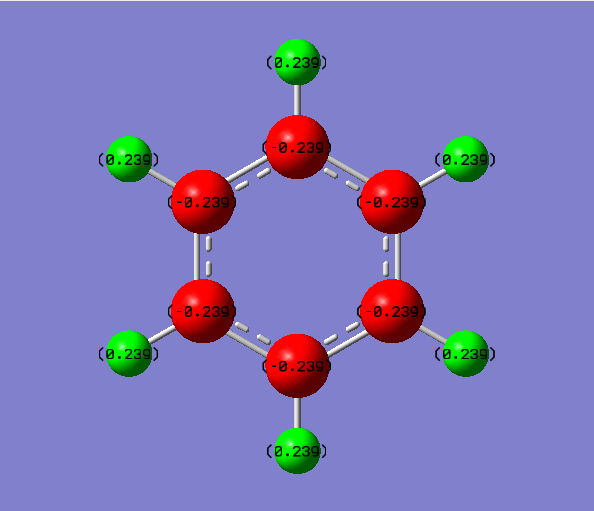
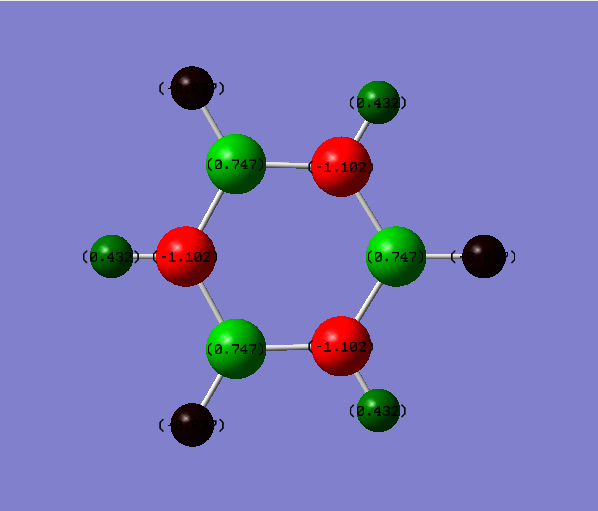
Charge comparison between aromatic systems
As seen below, while being isoelectronic, benzene and borazine have very different NBO charge distributions.Benzene Has a electronegative center caused by the delocalized pi system surrounding the carbon atoms. This results in all carbon atoms being equally electron dense (-0.239 arbitrary units) and all hydrogen atoms being equally less electron dense (+0.239 arbitrary units). Borazine is a different story, however, due to the large difference in electronegativity of boron and nitrogen in the central ring. Nitrogen is far smaller and electron dense than boron, causing it to be more electron rich (-1.102 arbitrary units) and drawing electron away from neighboring hydrogen atoms (+0.432 arbitrary units) and boron atoms (+0.747). The hydrogen atoms next to boron have a near neutral charge though, due to hydrogen and boron having similar Pauling electronegativity values.
Ng611 (talk) 15:52, 21 May 2018 (BST) Good discussion of charge distribution due to differences in electronegativity. I would also suggest including additional discussion on the similarities of charges on atoms related by symmetry, as well as the overall summation of the partial charges.
| Benzene | Borazine |
|
|
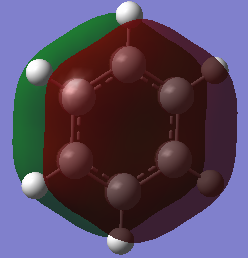
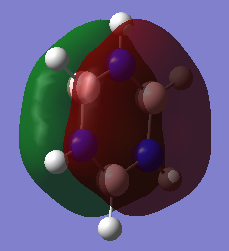
Comparison of Molecular orbitals between aromatic systems
Nature of Aromaticity
A common understanding or aromaticity is that in a planar ring system with 4n+2 electrons (Hückel's rule), each carbon atom will form a sigma bond to each other then form a conjugated pi bond above and below the ring. Each carbon will form equal distant bonds to each other longer than a double bond but shorter than a single bond. The enthalpy of the bonds will be above a single bond, but below a double bond. They will also resist reactions that would proceed rapidly with a double bond, requiring a species to activate the ring system. In addition, they will form a ring current when under the influence of an external magnetic field, leading to increased diamagnetic susceptability. These concepts of aromaticity taken from: https://onlinelibrary.wiley.com/doi/epdf/10.1002/chem.200700250 . Aromatic compounds do not have to contain carbon, as shown by borazine, which contains only boron, nitrogen and hydrogen. This concept can be seen in MO 17 of benzene and MO 17 of borazine, showing an electron cloud delocalised above and below the ring.
Overlapping P orbitals, however, do not provide a complete picture of aromaticity. The atomic orbitals combine to form a cloud completely covering the top and bottom of the ring, as shown by MO 17 of benzene and MO 17 of borazine, rather than forming a ring above and below the atoms on the ring. Some of the MOs of both compound represent linear combinations of atomic orbitals, while many are not combinations of atomic orbitals. The molecules form clear sigma bonds and pi bonds, with bonding and antibonding orbitals representing each, with some MOs representing neither. The P orbital overlap also breaks down quite clearly when observing the MOs of borazine, as the MOs show clear ionic character due to the difference in electronegativity of boron and nitrogen, showing distortions in the aromatic system drawing electron density to the nitrogen atoms.
Ng611 (talk) 15:56, 21 May 2018 (BST) I would expand on this second paragraph somewhat. If p-orbital delocalisation doesn't exclusively dictate aromaticity, then what does? How was the conceptual picture of aromaticity evolved? Your first paragraph contains an interesting discussion on experimental evidence though - well done.
Ng611 (talk) 15:59, 21 May 2018 (BST) You missed some opportunities for easy marks in the first section due to a few silly mistakes - remember to carefully check your work. Your explanation of aromaticity was good and your charge analysis was well performed and the report well laid out however. Well done.