Rep:Mod: 01338507
NH3 molecule
Optimisation information
Calculation method: RB3LYP
Basis set: 6-31G (d.p)
Final energy E(RB3LYP) in atomic units (au) : -56.55776873
RMS gradient: 0.00000485
Point group: C3V
Optimised N-H bond distance: 1.01798
Optimised H-N-H bond angle: 105.741
Item table
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES
Jmols of NH3
NH3 molecule |
The optimisation file is liked to here
Frequencies of NH3

The number of expected bands to appear on IR spectrum will be 2. Though the Infrared data shows that all 6 modes have dipole changes, the intensities of modes 4,5,6 are low that cannot be seen in the spectrum.
Vibrations information
Modes expected from the 3N-6 rule: 6 modes
Modes that are degenerate (ie have the same energy): Mode 2 and 3; Mode 5 and 6
Modes that are "Bending" vibrations: Modes 1, 2 and 3
Modes that are "Bond stretch" vibrations: Modes 4, 5 and 6
Mode that is highly symmetric: Mode 4
"Umbrella" mode: Mode 1
Number of bands expected to see in an experimental spectrum of gaseous ammonia: 2 bands
Atomic charges
N: -1.125 negative charge since N is more electronegative than H
H: +0.375 positive charge since H is less electronegative than N
Electronegativity: N=3.0 H=2.1
N2 molecule
Optimisation information
Calculation method: RB3LYP
Basis set: 6-31G (d.p)
Final energy E(RB3LYP) in atomic units (au) : -109.52412868
RMS gradient: 0.00000282
Point group: Dinfh
Optimised N-N bond distance: 1.10550
Optimised N-N bond angle: 180
Item table
Item Value Threshold Converged? Maximum Force 0.000005 0.000450 YES RMS Force 0.000005 0.000300 YES Maximum Displacement 0.000002 0.001800 YES RMS Displacement 0.000002 0.001200 YES
Jmols of N2
N2 molecule |
The optimisation file is liked to here
Frequency of N2

There is only 1 vibration mode for N2 molecule. No bands can be seen on the IR spectrum since there is no dipole changes when N2 molecule vibrates.
H2 molecule
Optimisation information
Calculation method: RB3LYP
Basis set: 6-31G (d.p)
Final energy E(RB3LYP) in atomic units (au) : -1.17853934
RMS gradient: 0.00007120
Point group: Dinfh
Optimised H-H bond distance: 0.74262
Optimised H-H bond angle: 180
Item table
Item Value Threshold Converged? Maximum Force 0.000123 0.000450 YES RMS Force 0.000123 0.000300 YES Maximum Displacement 0.000162 0.001800 YES RMS Displacement 0.000229 0.001200 YES
Jmols of H2
H2 molecule |
The optimisation file is liked to here
Frequency of H2

Like N2 molecule, there is only 1 vibration mode for H2 molecule. No bands can be seen on the IR spectrum since there is no dipole changes when H2 molecule vibrates.
Haber-Bosch Reaction Energy Calculation
Reaction equation: N2 + 3H2 → 2NH3
E(NH3)= -56.557769au
2*E(NH3)= -113.11554au
E(N2)= -109.52413au
E(H2)= -1.1785393au
3*E(H2)= -3.5356179au
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -0.0557921au
ΔE=-150kJ/mol
The ammonia product is more stable than the gaseous reactants since the overall reaction is exothermic.
H2O molecule
Optimisation information
Calculation method: RB3LYP
Basis set: 6-31G (d.p)
Final energy E(RB3LYP) in atomic units (au) : -76.41973726
RMS gradient: 0.00009507
Point group: C2V
Optimised O-H bond distance: 0.96539
Optimised H-O-H bond angle: 103.661
Item table
Item Value Threshold Converged? Maximum Force 0.000220 0.000450 YES RMS Force 0.000129 0.000300 YES Maximum Displacement 0.000711 0.001800 YES RMS Displacement 0.000737 0.001200 YES
Jmols of H2O
H2O molecule |
The optimisation file is liked to here
Frequency of H2O
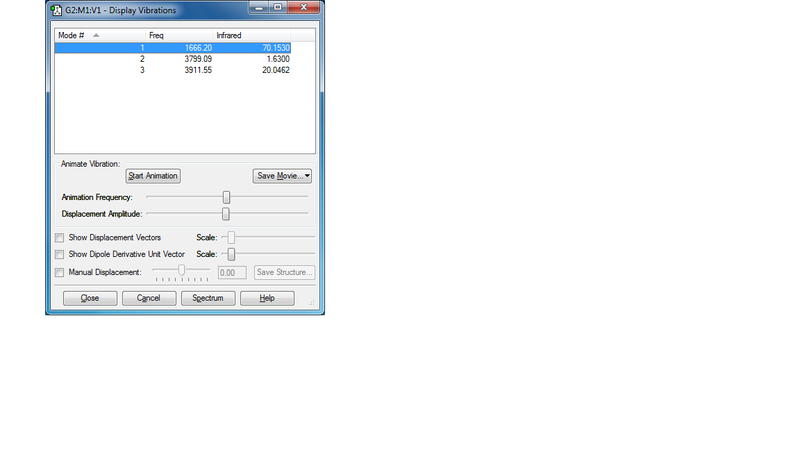
There are 3 vibration modes for H2O molecule. All of these modes show dipole changes. However, only 2 bands can be seen on IR spectrum since the intensity of Mode 2 is too low. Modes 1 and 3 will contribute to the bands.
Atomic charges
O: -0.943
H: +0.472
Molecular Orbitals

This MO is almost spherical and symmetrical. Oxygen 1s AO mainly contributes to this MO. As the energy of this MO is almost -19.14au, the orbital is very deep in energy compared to the following ones. It is occupied. However, as the orbital is held tightly by Oxygen and doesn’t show overlap with Hydrogen 1s orbitals, this orbital is not involved in bonding. Therefore, this MO is a non-bonding orbital.

This MO is also almost spherical and symmetrical. Oxygen 2s AO mainly contributes to this MO. The energy of this MO is around -0.10au, much higher in energy compared to MO1. It is occupied. As it is higher in energy and shows great extent of overlap with other AOs, this orbital is a bonding orbital.

This MO is symmetrical and shows large overlap between Oxygen p-orbital and Hydrogen 1s orbital. Oxygen p-orbital mainly contributes to this MO. The energy is around -0.515au, which means not very deep in energy. It is also occupied. As this MO shows great overlap, the AOs are in-phase with each other. Therefore, it is a bonding orbital.

This MO is asymmetrical but shows large overlap between Oxygen p-orbital and Hydrogen 1s orbital. Since the basis set in use is 6-31G (d.p), the p-orbital of Oxygen has some d-orbital character. Oxygen p-orbital mainly contributes to this MO. The energy is around -0.371au, which means not very deep in energy. It is also occupied. The large overlap and in-phase AO indicates it is a bonding orbital.

The MO is symmetrical. The shade indicates that AOs from Oxygen p-orbital and two Hydrogen 1s orbitals are out-of-phase with each other. Therefore, it is an anti-bonding orbital. Hydrogen 1s orbitals mainly contribute to this MO. The energy is around 0.151au, which means it is high in energy compared to the previous ones. The positive energy value also proves that it is an anti-bonding orbital. It is unoccupied.
Combustion of Hydrogen Reaction Energy Calculation
Reaction equation: H2 + 1/2O2 → H2O
E (H2O)= -76.419737au
E(O2)= -150.25742au
1/2*E(O2)=-75.128710au
E(H2)= -1.1785393au
ΔE=E(H2O)-[1/2E(O2)+E(H2)]=-0.1124877au
ΔE=-295kJ/mol
The literature value of enthalpy change for this reaction is -286kJ/mol.[1] As there is no much difference between predicted value and experimental value, the predicted enthalpy change using GaussianView is acceptable.
- ↑ Literature value of enthalpy change:https://www.bbc.com/education/guides/z8p72hv/revision/6
