Rep:Mod:ZhangWen
Introduction
The programme Gaussview was used to optimise all the molecules and was used to obtain the data presented below. The diagrams of MOs were also obtained using this programme.
NH3 Molecule
Optimisation
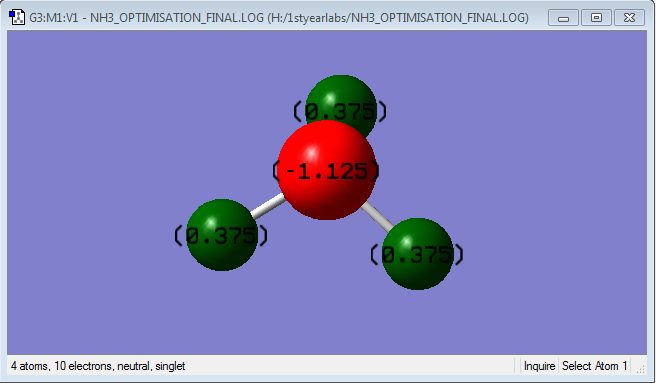
NH3 molecule |
NH3 Bond Distance = 1.10798
NH3 Bond Angle = 105.741
Calculation Method: RB3LYP
Basis Set: 6-31G(d,p)
Final energy E(RB3LYP) in atomic units (au): -56.55776873
RMS Gradient:
Point Group: C3V
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000072 0.001800 YES
RMS Displacement 0.000035 0.001200 YES
The optimisation file is linked to here
Vibrations and charges
Vibrations
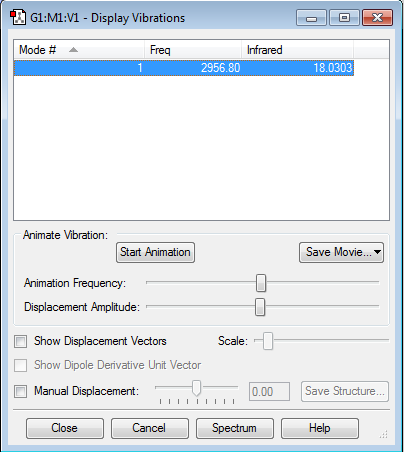
 Using the 3N-6 Rule to calculate the number of modes expected, 3(4)-6 = 6, 6 modes are expected. Modes 2 and 3 are degenerate and modes 5 and 6 are degenerate as they have the exact same frequencies. Modes 4,5 and 6 are stretching vibrations, while modes 1,2 and 3 are bending vibrations. Mode 4 is highly symmetric and Mode 1 is also known as the umbrella vibration. In an experimental spectrum of ammonia, 2 bands are expected to be seen. Vibrational mode 1 involves a very large change in dipole moment, resulting in a very large intensity of the peak. Vibrational modes 4,5 and 6 involve a small cahnge in dipole moment, resulting in a very weak peak which cannot be seen. Hence only the peaks from vibrational mode 1, 2 and 3 can be seen on the spectrum and since modes 2 and 3 are degenerate, only 2 bands are seen on a infrared spectrum.
Using the 3N-6 Rule to calculate the number of modes expected, 3(4)-6 = 6, 6 modes are expected. Modes 2 and 3 are degenerate and modes 5 and 6 are degenerate as they have the exact same frequencies. Modes 4,5 and 6 are stretching vibrations, while modes 1,2 and 3 are bending vibrations. Mode 4 is highly symmetric and Mode 1 is also known as the umbrella vibration. In an experimental spectrum of ammonia, 2 bands are expected to be seen. Vibrational mode 1 involves a very large change in dipole moment, resulting in a very large intensity of the peak. Vibrational modes 4,5 and 6 involve a small cahnge in dipole moment, resulting in a very weak peak which cannot be seen. Hence only the peaks from vibrational mode 1, 2 and 3 can be seen on the spectrum and since modes 2 and 3 are degenerate, only 2 bands are seen on a infrared spectrum.
Charges
Nitrogen is expected to have a negative charge and Hydrogen is expected to have a positive charge. Since Nitrogen is more electronegative than Hydrogen, the electrons are more attracted to the nitrogen atom and, resulting in a higher electron density around it and hence a negative charge develops, while a positive charge develops for each hydrogen atom.
N2 Molecule
Optimisation
N molecule |
N2 Bond Distance = 1.10424
Calculation Method: RB3LYP
Basis Set: 6-31G(d,p)
Final energy E(RB3LYP) in atomic units (au): -109.52412868
RMS Gradient: 0.00000060
Point Group: D∞H
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
The optimisation file is linked to here
Vibrations and charges
There will be no peaks as infrared spectroscopy only records vibrations which involve a change in its dipole moment. Since nitrogen exists as a homodiatomic molecule, a vibration would involve no change in its dipole moment and hence no peaks are seen. As nitrogen exists as a diatomic molecule with 2 nitrogen atoms with the same electronegativity, the charges on both atoms will be 0.
H2 Molecule
Optimisation
H2 molecule |
H2 Bond Distance = 0.74249
Calculation Method: RB3LYP
Basis Set: 6-31G(d,p)
Final energy E(RB3LYP) in atomic units (au): -1.17853936
RMS Gradient: 0.00000017
Point Group: D∞H
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES
The optimisation file is linked to here
Vibrations and charges
The vibrational mode 1 has a very high frequency as a hydrogen molecule has a very small reduced mass. Furthermore, no peaks are seen due to the same reason as the nitrogen molecule above. As hydrogen is an homodiatomic molecule and both atoms have the same electronegativity, both atoms have a charge of 0.
Haber-Bosch Process
The Haber-Bosch process is used industrially to manufacture ammonia from atmospheric nitrogen gas and hydrogen gas. The reaction is done using a metal catalyst and is conducted under high temperatures and pressures. The Haber-Bosch process is the main industrial scale method is produce ammonia used today due to its efficiency.
To determine the energy for the reaction N2 + H2 --> NH3, the following calculations are made:
E(NH3)= -56.55776873au
2*E(NH3)= -113.11553746au
E(N2)= -109.52412868au
E(H2)= -1.17853936au
3*E(H2)= -3.53561808au
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -113.11553746 - [(-109.52412868) + (-3.53561808)] = -0.0557907au = -146.47849401kJ/mol
Since the energy difference is negative, the ammonia product is more stable than the gaseous reactants
HCL
Optimisation
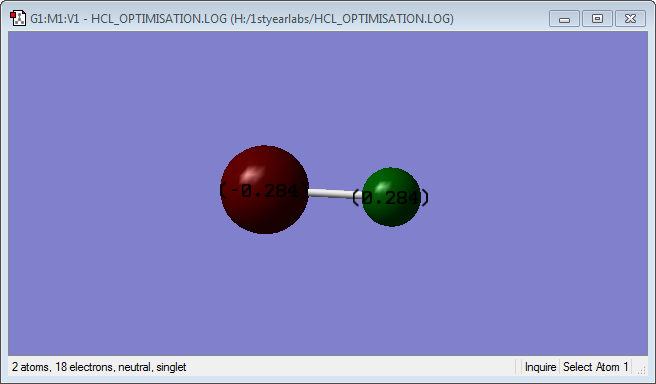
HCl molecule |
HCl Bond Distance = 1.28599
HCl Bond Angle = 180
Calculation Method: RB3LYP
Basis Set: 6-31G(d,p)
Final energy E(RB3LYP) in atomic units (au): -460.80077875
RMS Gradient: 0.00005211
Point Group: C∞V
Item Value Threshold Converged?
Maximum Force 0.000090 0.000450 YES
RMS Force 0.000090 0.000300 YES
Maximum Displacement 0.000139 0.001800 YES
RMS Displacement 0.000197 0.001200 YES
The optimisation file is linked to here
Vibrations and charges
Vibrations
For linear molecules, using the 3N-5 rule, one vibrational mode is expected. The vibrational mode is a stretching vibration.
Charges
Since Chlorine is more electronegative than hydrogen, the shared electrons are pulled towards the chlorine resulting in a higher electron density around the chlorine atom as compared to the hydrogen. Hence a partial negative charge and a partial positive charge develops around the chlorine and the hydrogen respectively.