Rep:Mod:ZY1416
NH3 Molecule
Summary
| Calculation Method | OPTF, RB3LYP |
| Basis Set | 6-31G(d,p) |
| RMS Gradient | 0.00000485 a.u. |
| E(RB3LYP) | -56.55776873 a.u. |
| Point Group | C3V |
Item Table
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES
The molecule
N-H bond distance = 1.01798 Angstrom
H-N-H bond angle = 105.741 degrees
NH3 molecule |
Analysis&Questions
![thumb]](/images/1/11/ZY1416_Display_Vibrations.png)
Q:how many modes do you expect from the 3N-6 rule?
A: N=4 3*4-6=6 modes
Q:which modes are degenerate (ie have the same energy)?
A: modes #2 and #3 are degenerate(H-N-H scissoring) and modes #5 and #6 are degenerate(N-H asymmetric stretching).
Q:which modes are "bending" vibrations and which are "bond stretch" vibrations?
A: modes #1,#2 and #3 are bending vibrations and #4,#5,#6 are stretching vibrations.
Q:which mode is highly symmetric?
A: mode #4 the stretching N-H symmetric streching
Q:one mode is known as the "umbrella" mode, which one is this?
A: mode #1 N-H wagging
Q:how many bands would you expect to see in an experimental spectrum of gaseous ammonia?
A: 4 bands
Charge Analysis
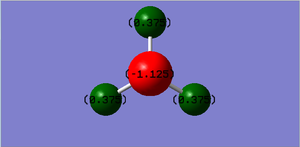
The charge on the N- atom is -1.125 and the charge on the H- atoms is 0.375.
It is expected that the nitrogen atom has the negative charge and the hydrogen atoms have the positive charge because nitrogen is more electronegative than hydrogen.
N2 Molecule
Summary
| Calculation Method | OPTF, RB3LYP |
| Basis Set | 6-31G(d,p) |
| RMS Gradient | 0.00978744 a.u. |
| E(RB3LYP) | -109.52404119 a.u. |
| Point Group | D∞h |
Item Table
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
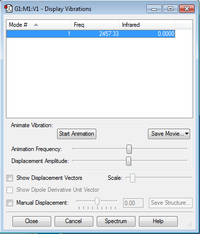
Frequency
It is a homonuclear molecule with one single vibration at frequency 2457.33 cm-1. And this vibration is IR-inactive as there is no change in dipole moment.
H2 Molecule
Summary
| Calculation Method | OPTF, RB3LYP |
| Basis Set | 6-31G(d,p) |
| RMS Gradient | 0.00005349 a.u. |
| E(RB3LYP) | -1.17853935 a.u. |
| Point Group | D∞h |
Item Table
Item Value Threshold Converged? Maximum Force 0.000093 0.000450 YES RMS Force 0.000093 0.000300 YES Maximum Displacement 0.000122 0.001800 YES RMS Displacement 0.000172 0.001200 YES
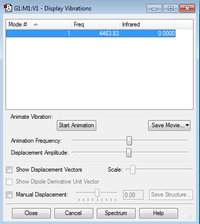
Frequency
It is a homonuclear molecule as well and it only has one vibration at frequency 4463.83 cm-1. And this vibration is IR-inactive as there is no change in dipole moment.
Haber-Bosch Reaction Energy Calculations
Calculated Result:
E(NH3)= -56.55776873 au and 2*E(NH3)= -113.11553746 au
E(N2)= -109.52404119 au
E(H2)= -1.17853935 au and 3*E(H2)= -3.53561805 au
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]
= -0.05587822 au
= -146.70826661 KJ/mol
(The ammonia product is thermodynamically more stable as the enthalpy change is negative.)
Investigation:
The calculated value for the standard enthalpy change of formation of ammonia is -73.354133305 KJ/mol but the experimental value is -45.8 KJ/mol[1]. This difference can arise because of the different calculation method used. The value obtained from Gaussian View is just an approximation.
SiH4
Summary
| Calculation Method | OPTF, RB3LYP |
| Basis Set | 6-31G(d,p) |
| RMS Gradient | 0.00000002 a.u. |
| E(RB3LYP) | -291.88802760 a.u. |
| Point Group | Td |
| Si-H Bond Length | 1.48485 A |
| H-Si-H Bond Angle | 109.471 degrees (Tetrahedral) |
Item Table
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
Information about the molecule
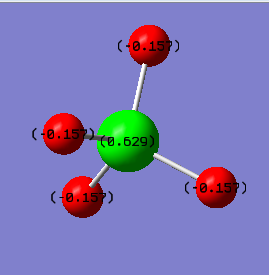
Charges: The charge on the Si atom is +0.629 and the charge on the four identical H atoms is -0.157.
Frequency: There are 9 vibrational modes as expected from the 3N-6 rule where N=5 for the SiH4 molecule. The scissor symmetric and stretching symmetric vibrations have zero infrared intensity because they are IR-inactive.
| Frequency/cm-1 | Infrared Intensity | Vibrational mode[2] |
|---|---|---|
| 919.21 | 136.1485 | scissor asymmetric |
| 919.21 | 136.1485 | scissor asymmetric |
| 919.21 | 136.1485 | scissor asymmetric |
| 978.78 | 0.0000 | H-Si-H scissor symmetric |
| 978.78 | 0.0000 | H-Si-H scissor symmetric |
| 2244.40 | 0.0000 | H-Si stretching symmetric |
| 2254.90 | 143.3961 | H–Si stretching asymmetric |
| 2254.90 | 143.3961 | H–Si stretching asymmetric |
| 2254.90 | 143.3961 | H–Si stretching asymmetric |
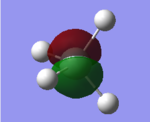
MO Analysis
These three filled MOs are degenerate i.e. they have the same energy -3.64 au. They are formed from the antibonding overlap between 2p orbitals.
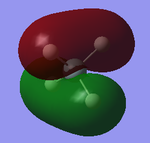
This is the HOMO(-0.35 au). It is formed from the bonding overlap between H 1s and Si 3p orbitals and the molecular orbital is 1t2. The energy gets much higher compared to the energy of MOs formed by core AOs.
This is the LUMO(0.05 au). It is formed from the antibonding overlap between H 1s and Si 3p orbitals and the molecular orbital is 2t2*.
Calculation
Formation of SiH4: Si(s) + 2 H2(g) ---> SiH4(g)
E(H2)= -1.17853935 au 2*E(H2)= -2.35707870 au
E(Si-H)= -291.88802760 au
ΔE=E(SiH4)-[2*E(H2)]= -289.5309489 au = -760163.5642431 KJ/mol
The experimental standard enthalpy change of formation of SiH4 is +34.31 KJ/mol[3] The calculated value is obtained from the formation of solid state SiH4 while the gaseous SiH4 is formed when determining the value of the standard enthalpy of formation. The differnce is because the sublimation of SiH4 is a very endothermic process.