Rep:Mod:ZQHMod3
In this module, the computational technique would be used to calculate the transition states of the reaction: the Cope rearrangement and Dields-Alder Cycloaddition. The geometry structure of the transition state will be found and so the corresponding low energy product.
The Cope rearrangement
Optimisation of 1,5 hexadiene Because of the free rotation of the C-C single bonds in the centre of the molecule, there are several possible structures for the molecule. They are 6 guache and 4 anti-periplanar conformers as lists in the Appendix 1 table. The molecules are drawn with Gaussian View 5. The different conformers are obtained by manually adjusting the dihedral angles of the molecule. Then they are “cleaned” by using the clean under Edit menu, followed by optimisation (HF/3-21G) with %memory = 250MB. After optimisation, the point group of the conformers are obtained by Symmetrize under the Edit menu.
| Conformer | Energy | Point Group | Geometry |
| anti1 | −231.692602 a.u. | C2 | Media:anti1 opt zqh.ogg |
| anti2 | −231.692535 a.u. | Ci | Media:anti2 opt zqh.ogg |
| anti3 | −231.689071 a.u. | C2h | Media:anti3 opt zqh.ogg |
| anti4 | −231.690971 a.u. | C1 | Media:anti4 opt zqh.ogg |
| guache1 | −231.687716 a.u. | C2 | Media:guache1 opt zqh.ogg |
| guache2 | −231.691667 a.u. | C2 | Media:guache2 opt zqh.ogg |
| guache3 | −231.692661 a.u. | C1 | Media:guache3 opt zqh.ogg |
| guache4 | −231.691530 a.u. | C2 | Media:guache4 opt zqh.ogg |
| guache5 | −231.689616 a.u. | C1 | Media:guache5 opt zqh.ogg |
| guache6 | −231.689160 a.u. | C1 | Media:guache6 opt zqh.ogg |
From the table above, the anti1, anti2 and guache3 are the most stable structures. They are optimised with B3LYP/DFT/6-31G(d) setup. Guache3 is the most stable one from the table above.
| Conformer | Energy | Point Group | Geometry |
| anti1 | −234.61276 a.u. | C2 | Media:anti1 opt 631g zqh.ogg |
| anti2 | −234.61267 a.u. | Ci | Media:anti2 opt 631g zqh.ogg |
| guache3 | −234.61230 a.u. | C1 | Media:guache3 opt 631g zqh.ogg |
The new results of the three structures from higher level basis are lower than the previous ones. All the minimum energy values are about 3 a.u. lower. The anti1 conformer is the most stable one while guache3 is the least one, which is different from the previous order. The reason for anti1 is the most stable conformer is the best overlap between σ C-H and σ* C(3)-C(4) orbital.
Frequency Analysis
The frequency analysis of the three conformers are run from the optimised B3LYP/DFT/6-31G(d) structures. The low frequencies below shows the optimisation of the conformers is successful.
Anti1
Low frequencies --- -9.2657 -5.2822 -0.0004 0.0004 0.0005 23.6509 Low frequencies --- 73.2248 96.1113 113.5387
Anti2
Low frequencies --- -13.8019 -8.9932 -0.0004 -0.0001 0.0001 3.5924 Low frequencies --- 71.6349 78.0954 116.2414
Guache3
Low frequencies --- -7.0386 -3.6641 -0.0006 -0.0004 -0.0002 9.9938 Low frequencies --- 71.8472 98.9932 122.3847
The table shows the C=C symmetric and asymmetric stretch frequencies. The symmetric stretches of the two anti conformers have a low intensity due to the high symmetry of the conformer. They belong to a high symmetry point group. The guache3 belongs to a low symmetry group, so both of the symmetric and asymmetric stretches have a higher intensity values.
Thermochemical data
The data are from the .log files of the vibration analysis.
The meanings of the terms are
1 the potential energy at 0 K including the zero-point vibrational energy (E = Eelec + ZPE)
2 the energy at 298.15 K and 1 atm of pressure which includes contributions from the translational, rotational, and vibrational energy modes at this temperature (E = E + Evib + Erot + Etrans)
3 the third contains an additional correction for RT (H = E + RT) which is particularly important when looking at dissociation reactions,
4 the last includes the entropic contribution to the free energy (G = H - TS)
Each type of the energies of anti1 conformer is the lowest among the three structures. The energy values of anti2 are close to anti1, but for guache3, the energy differences are more.
Anti1
Sum of electronic and zero-point Energies= -234.470413 Sum of electronic and thermal Energies= -234.463138 Sum of electronic and thermal Enthalpies= -234.462194 Sum of electronic and thermal Free Energies= -234.501319
Anti2
Sum of electronic and zero-point Energies= -234.470332 Sum of electronic and thermal Energies= -234.463025 Sum of electronic and thermal Enthalpies= -234.462081 Sum of electronic and thermal Free Energies= -234.501962
Guache3
Sum of electronic and zero-point Energies= -234.469842 Sum of electronic and thermal Energies= -234.462653 Sum of electronic and thermal Enthalpies= -234.461709 Sum of electronic and thermal Free Energies= -234.501306
Optimisation of Transition states
Optimise Chair TS with Computing the Force Constant Matrix
An allyl fragment (CH2CHCH2) is drawn and optimised with HF/3-21G basis. The fragment is then copied with Append Molecule function so two allyl fragments are in one molecules window. The two fragments are 2.2A apart.
Opt+Freq and Optimization to a TS (Berny) are selected. Basis setup is HF/3-21G. The calculation will be done once. Additional keyword is Opt=NoEigen. With these setup, the optimised structure of the Chair TS is found.
| Frequency | Intensity | Energy | .gif |
| −818 | 5.91 | −231.61932 a.u. | 
|
Optimise Chair TS with Freezing Reaction Coordinates
The distance apart from the two terminal carbon pairs of the two fragment are fixed as 2.2A by Redundant Coord Editor under the Edit menu. The optimisation is done to find the minimum energy geometry with HF/3-21G. Then with setup Opt+Freq, Optimization to a TS(Berny) and HF/3-21G, the pre-optimised .chk file is optimised with fixed bond length.
| Frequency | Intensity | Energy | .gif |
| −818 | 5.85 | −231.61932 a.u. | 
|
Optimising the Boat TS with QST2 method
Firstly, two anti2 conformer molecules are added into one molecule window and the carbon atoms of the 2 molecules are labeled by using the Atom List under Edit menu, as one is reactant, the other is product. The molecule is optimised with Opt+Freq, TS(QST2) and HF/3-21G with additional keywords, Opt=NoEigen. The optimisation is failed since the structures of the molecules are chair form.
The structure is then manually adjusted. The dihedral angle of C2-C3-C4-C5 is change to 0 degree, and the bond angle C2-C3-C4 and C3-C4-C5 are changed to 100 degree for the reactant molecule. Then for the product molecule, same thing is done. The new structure is sent to optimise again to obtain a successful optimised structure.
| Frequency | Intensity | Energy | .gif |
| −840 | 1.64 | −231.60280 a.u. | 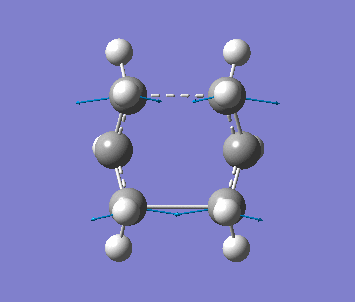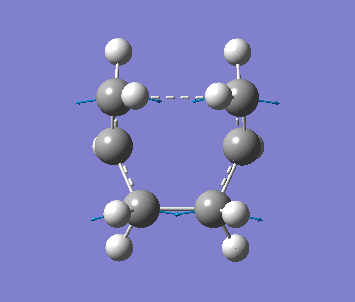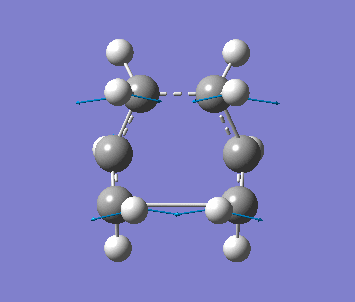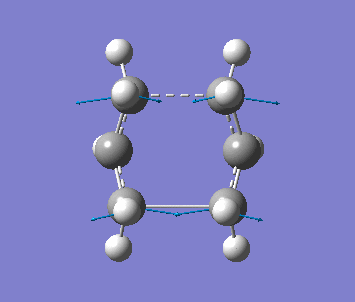
|
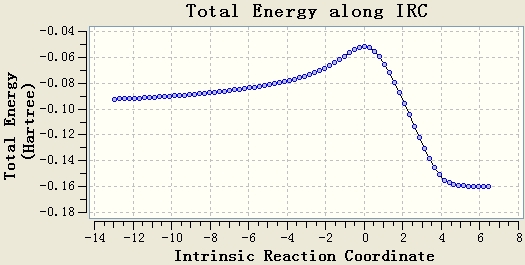
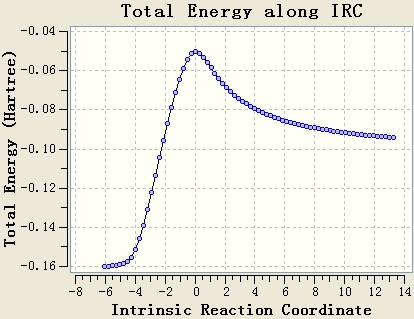
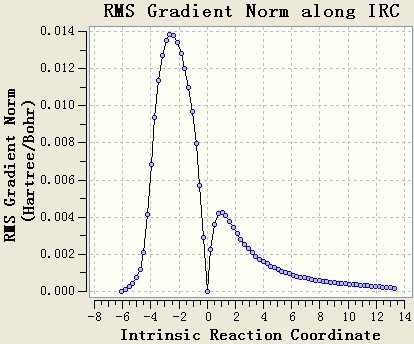
Intrinsic Reaction Coordinate
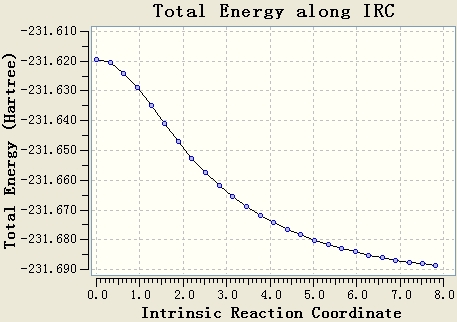
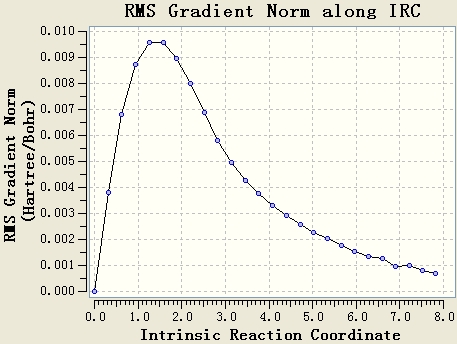
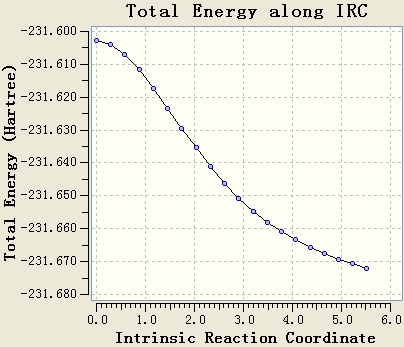
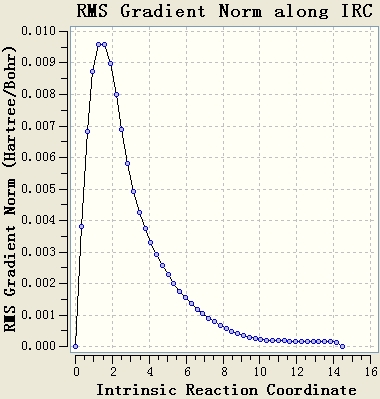
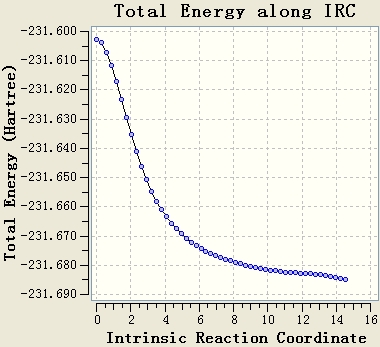
The IRC calculation is done with the setups IRC, HF/3-21G, maximum steps=50 and force constant will be calculated once. The resultant table shows that the optimisation is not end at a minimum energy state, since the gradient of the energy curve at the end is not 0.
Chair transition state results. Energy = -231.61932 a.u..
Boat transition state results. Energy = -231.60280 a.u..
The original IRC optimisation stops at 23 steps, so increasing the number of steps and re-run the IRC are meaningful. This will work if the maximum 50 steps is not enough.
Re-run the IRC with force constants calculated at each step is a good method. From the table, it can be seen that the gradient of the energy curve at the end is 0. So the geometry is optimised well.
Chair transition state results. Energy = -231.61932 a.u..
Boat transition state results. Energy = -231.60280 a.u..
The final IRC is done to give the following structure.
Activation energy
The activation energy of the cope rearrangement is calculated with two different bases, HF/3-21G, and B3LYP/631G(d). The activation energy is obtained by subtract the reactant energy from the transition state energy. Since the lowest energy conformer of the two bases is different, the reactant conformer used is different.
| Basis | Reactant energy | Chair TS energy | Boat TS energy | Activation energy to Chair TS | Activation energy to Boat TS | Conformer chosen |
| HF/3−21G | −231.69266 a.u. | −231.61932 a.u. | −231.60280 a.u. | 0.07334 a.u. | 0.08986 a.u. | Guache3 |
| B3LYP/6−31G(d) | −234.61276 a.u. | −234.42750 a.u. | −234.54639 a.u. | 0.18526 a.u. | 0.06637 a.u. | Anti1 |
The results from the two diferent basis are opposite. The 6-31G(d) basis suggests that the activation energy of boat structure is smaller which is unexpected. Also 6-31G(d) is a higher basis.
From the results of 3-21G basis, it can be seen that the activation energy of boat TS is larger than that of chair TS, so the chair TS is kinetic favoured.
Conclusion
From the above analysis, the guache3 conformer and the anti1 conformer are the most stable conformers. It depends on the basis used. The activation energy of chair TS is lower than that of boat TS, so the chair TS is the major under kinetic condition.
The Diels-Alder Cycloaddition
Optimisation of Ethene and Butadiene
The reactants, ethene and butadiene, are drawn with Gaussian View and then optimised with AM1 method.
| Reactants | LUMO | HOMO | Energy |
| Ethene | 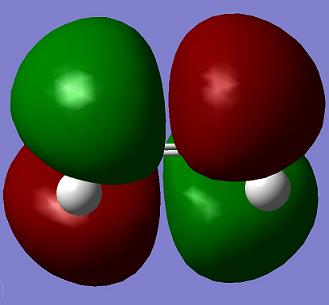
a |

s |
0.026190 a. u. |
| cis-Butadiene | 
s |

a |
0.048797 a. u. |
Orbital symmetry is very important in cycloaddition reaction. It can be seen from the table easily that the HOMO of ethene is s and LUMO of cis-butadiene is a. Also LUMO of ethene is a and HOMO of cis-butadiene is s. So the cycloaddition reaction is allowed.
Optimisation of Transition states
The transition state of the reaction is optimised by using the freezing coordinate method. The basis set used is AM1. The guess structure of the transition state is from the figure below. The guessed distance between the bonding atoms are 2.09A.
| LUMO | HOMO | HOMO−1 | Energy | .gif | Frequency |

|

|
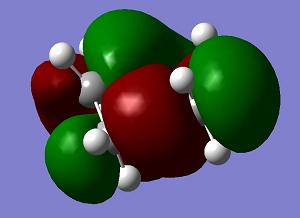
|
0.20111 a. u. | 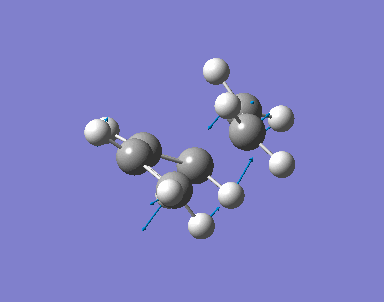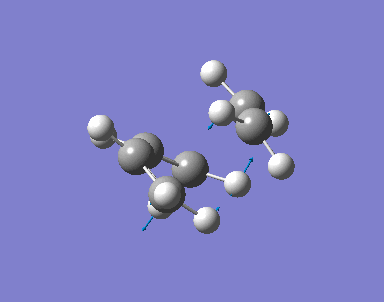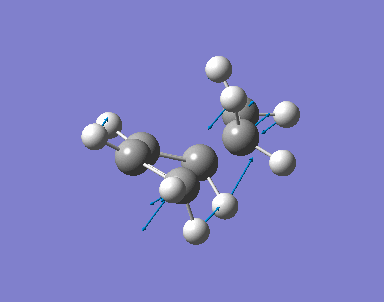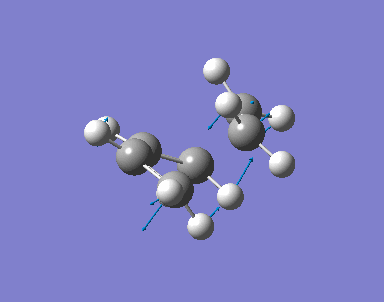
|
−944 |
The LUMO and HOMO-1 of the transition state are symmetric, and the HOMO of the transition state is asymmetric. The energies of HOMO and HOMO-1 are very close. Though HOMO is an anti bonding orbital, the structure is stabilised.
Intrinsic Reaction Coordinate
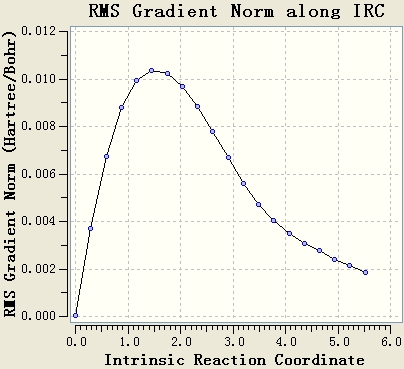
The IRC is done by using AM1 basis. The force constant is calculated at every step and both directions are calculated.
The gradients of curves of both directions are very close to 0. The optimised transition state is at low energy level.
Optimisation of Cyclohexadiene and Maleic Anhydride
The reactants, Cyclohexadiene and Maleic Anhydride, are drawn with Gaussian View and then optimised with AM1 method.
| Reactants | LUMO | HOMO | Energy |
| Cyclohexadiene | 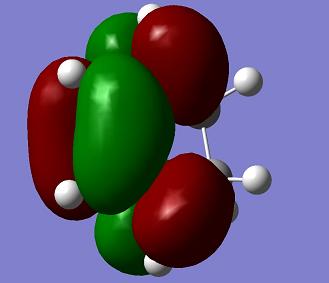
s |
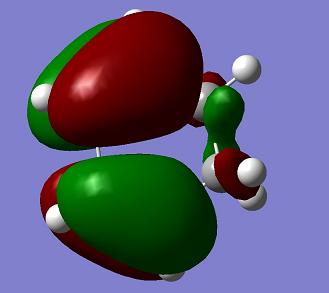
a |
0.027711 a. u. |
| Maleic Anhydride | 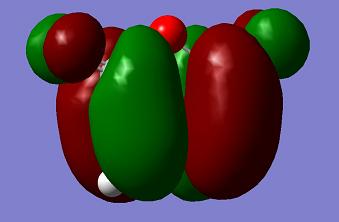
a |
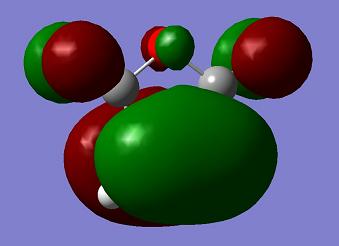
s |
−0.12182 a. u. |
The orbital symmetry of the two reactants is like the previous case. The HOMO of maleic anhydride and LUMO of cyclohexadiene are the same symmetry and so are the LUMO of maleic anhydride and HOMO of cyclohexadiene.
Optimisation of Transition states
The transition state of the reaction is optimised by using the freezing coordinate method. The basis set used is AM1. Due to the repulsion from the (O=C)-C-(C=O) fragement and the CH2-CH2 or two alkene fragments, the guessed structure need to be adjusted manually. Otherwise the optimisation will fail and no product forms. For the exo product, the dihedral angles of cyclohexadiene are set to be -12.8 for C=C-C=C and ±35.9 for two C-C=C-C, the bonding atoms distances starts from 2.4-2.6A and are fixed at 2.09A. For the endo product, since the repulsion is much smaller, the distance between bonding atoms are from 2.10-2.11 and are fixed at 2.09A. No dihedral angle needs to be changed.
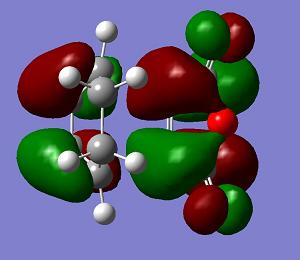
| LUMO+1 | LUMO | HOMO | HOMO−1 | Energy | .gif | Frequency |

s |
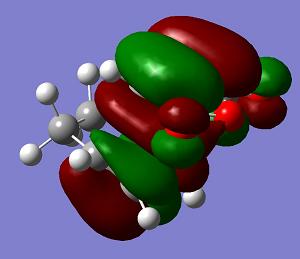
a |
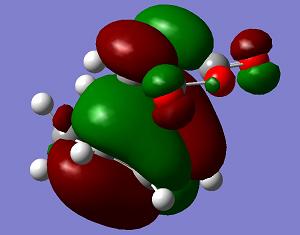
s |
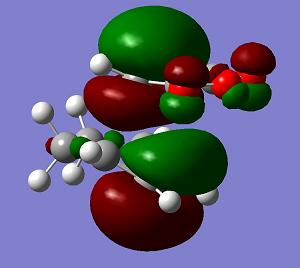
a |
−0.051505 a. u. | 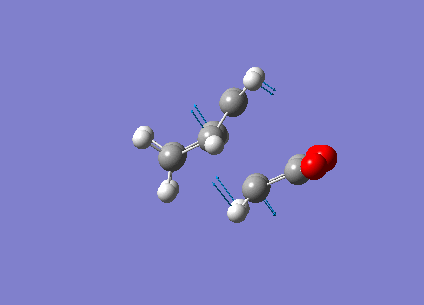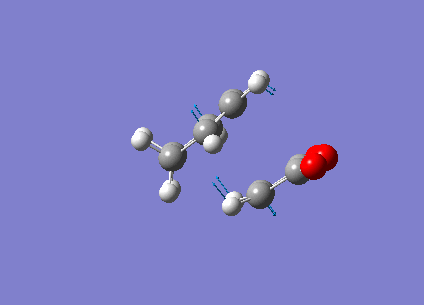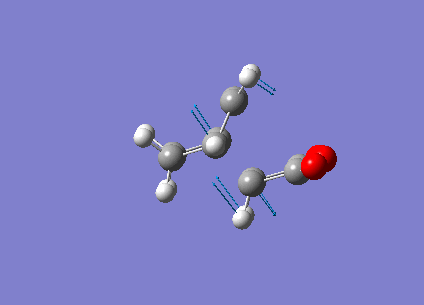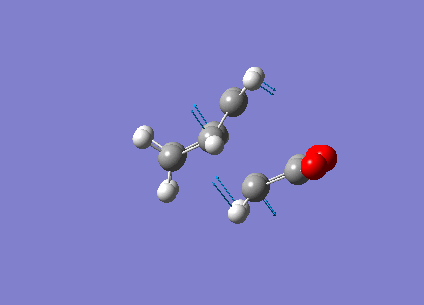
|
−860 |
| LUMO+1 | LUMO | HOMO | HOMO−1 | Energy | .gif | Frequency |

s |
a |
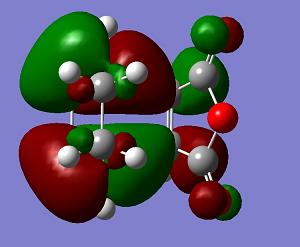
a |
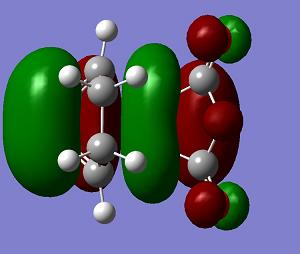
s |
−0.050420 a. u. | 
|
−812 |
The endo transition state structure has a lower energy than that of exo as expected. The endo product is the major at low temperature, since the energy gap between the transition state and reactant is smaller. The endo product is kinetic favoured. The orbitals of the exo and endo transition states are similar. Only LUMO+1 shows a difference. So the difference in energy is due to the steric effect as mentioned.
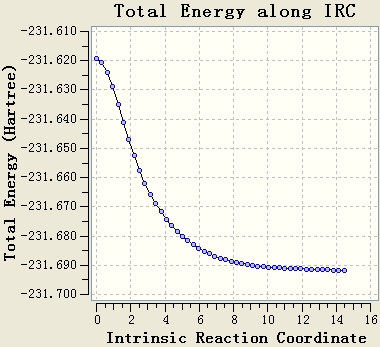
Intrinsic Reaction Coordinate
The IRC is done by using AM1 basis. The force constant is calculated at every step and both directions are calculated. Both the IRCs give good results with the gradients of energy curves being close to 0.
Discussion
Bond length
| Bond Type | C-C | C=C | forming C-C | Typical C-C | Typical C=C |
| Bond Length | 1.39A | 1.38A | 2.09A | 1.54A | 1.34A |
| Bond Type | C=C in cyclohexadiene | C-C in cyclohexadiene | C=C in Maleic anhydride | forming bond | Transition state | Typical C-C bond | Typical C=C bond |
| Bond Length | 1.39A | 1.40A | 1.41A | 2.16A | Endo | 1.54A | 1.34A |
| Bond Length | 1.39A | 1.39A | 1.41A | 2.17A | Exo | 1.54A | 1.34A |
From the bond length listed in the table, the forming bond of transition state structure is longer than the typical C-C bond, which is 1.54A. The C=C bonds of the transition states are also longer, since the typical C=C bond length is 1.34A. The longer forming bond length suggests that the interaction is attractive. The two reactant molecules coming together and form the product. The longer C=C bond length indicates that the delocalisation of electron density. The C-C bond length is shorter than the typical C-C bond length and is nearly the same length as the C=C bond. In the transition state, the C=C bonds and C-C bonds are in a situation between forming and breaking.
Compare the endo and exo transition states, the bond length of formed bonds are nearly same, but the bond length of forming bonds are a little different. That of endo structure is shorter, so the endo structure is favourable. It is closer to the typical C-C bond length.
Activation energy
| Basis | Reactant energy | Endo TS energy | Exo TS energy | Activation energy to Endo TS | Activation energy to Exo TS |
| AM1 | −0.09411 a. u. | −0.05150 a. u. | −0.05042 a. u. | 0.04261 a. u. | 0.04369 a. u. |
The activation energy of endo structure is lower than that of exo structure, so endo structure is kinetic favoured.
Conclusion
From the optimisation of the reactants, the symmetry of the orbitals allows the reaction to take place. The HOMO of one reactant has the same symmetry as the LUMO of the other reactant.
For both of the reactions, the forming bond length of the transition states indicates the attractive reaction. The endo structure has a lower energy level and so lower activation energy. It is the kinetic favoured route. The reason for this is mainly due to the repulsion between CH2-CH2 and O=C-C-C=O fragments during the approach of the two reactant while form the exo TS.
Conclusion
From the analysis above, the computational technique can predict the transition state of simple reactions. For complex reactions, higher basis set will be used and stronger computer power is needed. The guessed transition state structure is very important. A good starting point will save much resource during the calculation of correct transition states and their energies.
Reference
1 http://hdl.handle.net/10042/to-13844
2 http://hdl.handle.net/10042/to-13845
3 http://hdl.handle.net/10042/to-13846
4 http://hdl.handle.net/10042/to-13847
5 http://hdl.handle.net/10042/to-13848
6 http://hdl.handle.net/10042/to-13849
7 http://hdl.handle.net/10042/to-13850
8 http://hdl.handle.net/10042/to-13851
9 http://hdl.handle.net/10042/to-13853
10 http://hdl.handle.net/10042/to-13854
11 http://hdl.handle.net/10042/to-13855
12 http://hdl.handle.net/10042/to-13856
13 http://hdl.handle.net/10042/to-13857
14 http://hdl.handle.net/10042/to-13858
15 http://hdl.handle.net/10042/to-13859
16 http://hdl.handle.net/10042/to-13860
17 http://hdl.handle.net/10042/to-13861
18 http://hdl.handle.net/10042/to-13862
19 http://hdl.handle.net/10042/to-13863
20 http://hdl.handle.net/10042/to-13864
21 http://hdl.handle.net/10042/to-13865
22 http://hdl.handle.net/10042/to-13866
23 http://hdl.handle.net/10042/to-13867
24 http://hdl.handle.net/10042/to-13868
25 http://hdl.handle.net/10042/to-13871
26 http://hdl.handle.net/10042/to-13872
27 http://hdl.handle.net/10042/to-13873
28 http://hdl.handle.net/10042/to-13874
29 http://hdl.handle.net/10042/to-13875
30 http://hdl.handle.net/10042/to-13876
31 http://hdl.handle.net/10042/to-13878
32 http://hdl.handle.net/10042/to-13877
33 http://hdl.handle.net/10042/to-13879
34 http://hdl.handle.net/10042/to-13880