Rep:Mod:ZL8215 transition state
Introduction

Reactants must be given enough energy for them to overcome a reaction activation barrier to start a reaction. The highest energy along a reaction coordinate is a transition state (TS). At transition state, the possibility of the reaction goes into reactants or product is 50:50. The plot of chemical species' energy versus reaction coordinates is the potential energy surface. In 3D, it is the potential energy landscape. Mountain ranges can be an analogy to the potential energy landscape. There are many minima which can be reactants or products as they are stable and have a low energy geometry. The peaks are the energy barriers, where some of them can be the transition states of some reactions.
Nf710 (talk) 10:30, 15 December 2017 (UTC) Careful, The PES is not restricted to 3D. And there is only 1 reaction coord. the rest of the dimensions in Gaussian are the degrees of freedom.
In this wiki, the reaction energies of 3 pericyclic reactions were studied using Gaussview. There are different methods to find the energy of reactants, TS and products. In the first two exercises, the TS of the reaction was carefully drawn and adjusted then optimised at either PM6 or B3LYP/6-31G, later the reactants and products geometries were found using IRC calculation. In the third exercise, the product was drawn and optimised then by breaking the bond, the optimised TS was found and the reactant geometry was found from IRC calculation. Afterwards, a reaction energy profile was drawn, the activation energy barrier ( the free energy of TS - the free energy of reactants ) and the change of enthalpy of a reaction (the free energy of product - the free energy of reactants) were calculated and compared.
A frequency calculation was run for the optimised TS to confirm the geometry is at TS. In the frequency calculation, one imaginary frequency (a negative value) appears when it is at the transition state (TS). This is because TS is on a saddle point, it has a positive and a negative value of the second derivative in 3D. The second derivative (the curvature) is a negative value when it is a peak and is a positive value when it’s a minimum. Figure 1 illustrates that if there are two reaction pathways that a reactant can take, the reactant should take path A for the lowest energy reaction, so point A is the correct TS. At point A, there is only one negative value for the second derivative, whereas at point B, there are 2 negative values of the second derivative.
Nf710 (talk) 10:30, 15 December 2017 (UTC) You seem to understand, just be careful as Gaussian does with in 3n-6 dimensions.
Since it is a plot of energy, the first derivative gives the force while the second derivative gives the force constant. The frequency (f) and the force constant (k) are related by the equation: f=1/2π √(k/m) where m is the reduced mass: f=1/2π √(k/m) where m is the reduced mass.
[4+2] Cycloaddtion of 1,3-Butadiene and Ethylene
Reaction Scheme
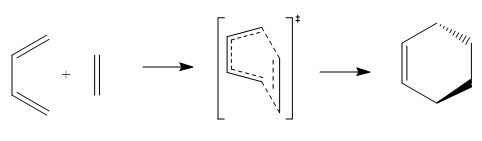
| Trans-1,3-butadiene | Cis-1,3-butadiene | Ethylene | Transition State | Cyclohexene | ||||||||||
|
Transition State Analysis
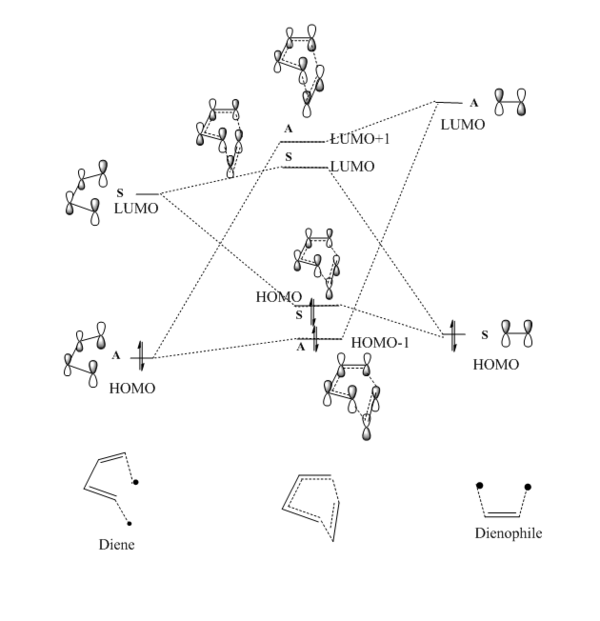
MO Analysis
(Fv611 (talk) Very good MO diagram, even though the position of the ethene LUMO seems a bit too low in energy. Well done on relating the higher energy of the MOs with the fact that they correspond to a TS.)

| HOMO of Ethylene | LUMO of Ethylene | HOMO of Butadiene | LUMO of Butadiene |
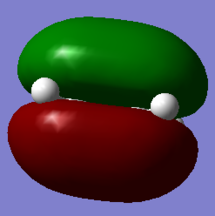
|
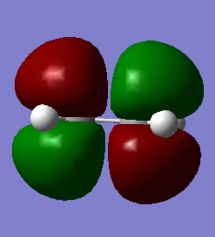
|

|
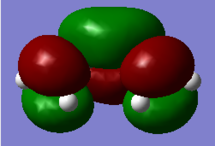
|
| HOMO-1 of TS | HOMO of TS | LUMO of TS | LUMO+1 of TS |

|

|
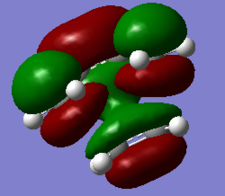
|

|
Atomic orbitals (AO) with the same symmetry can mix to form molecular orbitals (MO). The HOMO of dienophile and the LUMO of diene are both symmetric (S), they combine and form symmetric MOs (HOMO and LUMO of TS) while the LUMO of dienophile and the HOMO of diene are antisymmetric (AS), they combined and formed antisymmetric MOs (HOMO-1 and LUMO+1 of TS). (Figure 3) Since the MO of the transition state were drawn, the energy of MO of TS are higher than the energy of reactants and product.
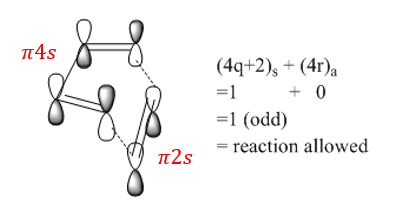
Another way to identify if a pericyclic reaction is allowed or not is to use Woodward-Hoffmann rules. In this [4+2]-cycloaddition reaction between 1,3-butadiene and ethylene, the total number of (4q + 2)s and (4r)a components is odd. Therefore, the reaction is allowed. (Figure 4)
The interaction of the LUMO of dienophile and the HOMO of diene is stronger than the interaction of the HOMO of dienophile and the LUMO of diene, as the energy gap is smaller so better overlap of AOs. Therefore, the MOs splitting is larger. Orbital overlap integral gives how much two orbitals overlap with each other. When 2 AOs are entirely overlapping, the integral equals to 1; When there is no overlapping, the integral equals to zero. S-S interaction and AS-AS interaction have non-zero overlap integral while S-AS interaction has a zero overlap integral.
Bond Length Analysis
| Reactants | TS | Product |

|

|
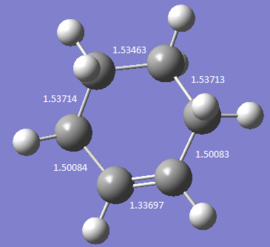
|
As the reaction processes, the double bonds increase in length and the single bonds decrease in length. In the reaction, 3 C=C bonds were broken with 1 C=C bond reformed and 2 C-C bonds formed. The product has a typical bond length for sp3-sp3 C-C bond (1.54 Å) and sp2-sp3 C-C bond (1.50 Å) and sp2-sp2 C=C bond (1.34 Å). The Van Der Waal’s radius of a C atom is 1.7 Å , this is the radius of a C atom when it does not bonded to another C atoms. All the bond lengths between Cs in this reaction is less than 3.4 Å which means all neighbouring carbon atoms have interactions between each other. The covalent radius is the radius of an atom when it forms a covalent bond, which is 0.76 Å for a sp3 C and 0.73 Å for a sp2 C. The sum of these two covalent radius match with the bond length of the newly formed C-C bond in the product of this reaction.
(Fv611 (talk) Remember that we ask to compare bond distances with VdW radii specifically for the bond that is being formed.)
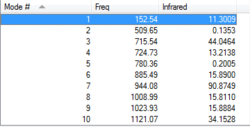
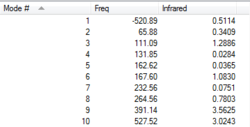
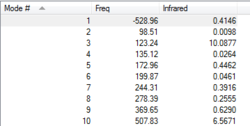
Transition State Vibration Analysis
Reaction of Cyclohexadiene and 1,3-Dioxole
Reaction Scheme

This is cycloaddition reaction, there are 2 products formed due to different approaches, the exo product and the endo product. Theoretically, it is predicted that endo product is more favoured than the exo product. Here, the reactant, TS and products of both reactions were optimized at B3LYP level and energy profile was plot in order to see which product is more favourable. All the TS have a negative value in their frequency calculation and all reactants and products have positive frequency values.
MO Analysis
(Fv611 (talk) Good MO diagram, but your discussion is missing any reference to the difference between exo and endo cases.)
| MO structure of EXO TS | |||
|---|---|---|---|
| HOMO-1 of EXO TS | HOMO of EXO TS | LUMO of EXO TS | LUMO+1 of EXO TS |
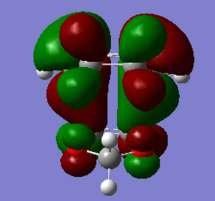
|

|

|
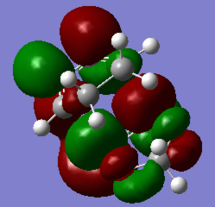
|
| MO structure of ENDO TS | |||
| HOMO-1 of ENDO TS | HOMO of ENDO TS | LUMO of ENDO TS | LUMO+1 of ENDO TS |
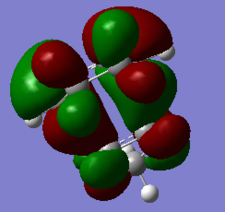
|
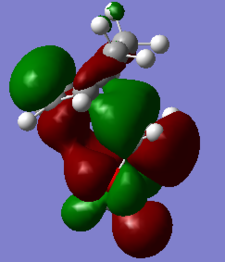
|
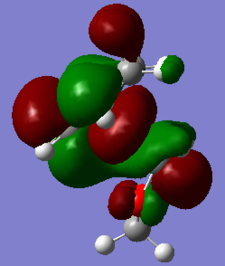
|
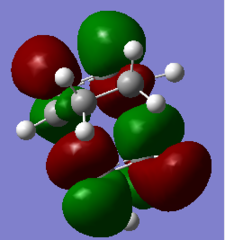
|
This is an inverse demand Diels-Alder (DA) reaction (both endo and exo reactions). A normal DA reaction (e.g. exercise 1), the diene has greater electron density and donates electrons to the LUMO of diene. Therefore, the HOMO of diene and the LUMO of dienophile have closer energy levels and interact strongly in a normal DA reaction. However, in this reaction, the dienophile has a higher electron density because O is an electron rich atom which increase the electron density of the dienophile. This makes the dienophile donates electrons to the diene LUMO. Therefore, on the MO graph (Figure 7), the HOMO of dienophile and the LUMO of the diene interact strongly. (P.s. the energy level of the MOs matches with the calculation results)
Nf710 (talk) 10:40, 15 December 2017 (UTC) You can show this analytically by looking at the energys of the MOS of the reactants in the same potential energy surface. But yes you can also see it qualitatively by looking at the MOS.
Energy Barrier analysis

| Components | Energy/Hatress | Energy/kJmol-1 |
|---|---|---|
| cyclohexadiene | -233.324373 | -612592.8718 |
| 1,3-dioxole | -267.068642 | -701188.411 |
| Sum of Reactants energy | -500.393015 | -1313781.283 |
| endo TS | -500.33215 | -1313621.482 |
| exo TS | -500.329168 | -1313613.653 |
| endo product | -500.418691 | -1313848.695 |
| Exo product | -500.417323 | -1313845.103 |
| Components | Activation Barrier/kJmol-1 | Enthalpy Change /kJmol-1 |
|---|---|---|
| Endo | 159.8009872 | -67.41230834 |
| Exo | 167.6302247 | -63.82062592 |
From Figure 8, it can be seen that both the endo TS and product have a more negative free energy value than the EXO TS and product. Table 7 shows that the exo TS has a greater activation barrier than endo TS, which means forming an endo product is faster than forming an exo product, so endo product is kinetically favoured. This is due to the secondary orbital overlapping between pi orbital on O and the LUMO of diene stabilises the endo transition state, whereas the exo TS does not have this stabilisation.
The endo product is more stable than the exo product, so endo product is more thermodynamically favoured. This is because that the exo product is more sterically hindered due to the steric clash between the bridging of sp3 carbons and the O-C-O ring whereas for endo product, the 2 rings are in different faces, so steric clash is prevented. Therefore, endo product is both thermodynamically and kinetically favoured. The products (both endo and exo) of this reaction is more stable than the reactants, therefore the change of enthalpy is negative and it’s an exothermic reaction.
Nf710 (talk) 10:44, 15 December 2017 (UTC) This is good your energies are correct and you have come to the correct conclusions. You coudl have shown the sterics with a diagram. Also your intro was quite brief, some understanding of the quantum mechanical methods would have been nice.
Secondary Overlap
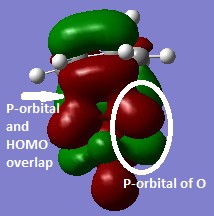 |
There is a secondary orbital overlapping between the p-orbital of oxygen and the antibonding orbital of diene, which stabilises the endo transition state, lowers the energy of endo TS, allows the endo product to be kinetically favourable. The exo product dose not have the secondary overlap. Figure 9 is a back view of the endo TS HOMO. |
Diels-Alder vs Cheletropic
Reaction Scheme
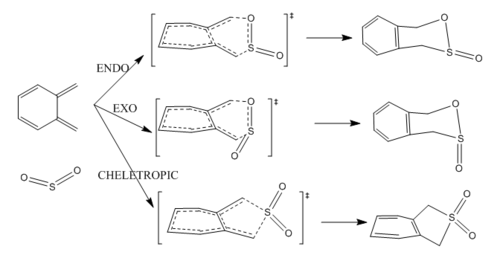
The pericyclic reaction of SO2 and o-xylylene can have three reaction pathways. In general, it is a competition between Diels-Alder reaction and Cheletropic reaction. For DA reaction, it is the S=O bond of SO2 interacting with the pi system of the diene while for cheletropic reaction, it is the lone pair on S of SO2 interacting with the pi system of diene. ALL chemical species were optimized at PM6 level to find their energy and a energy profile was plotted to study which pathway is more favourable.
| Xylylene | SO2 | Endo TS | Exo TS | Cheletropic TS | Endo product | Exo product | Cheletropic product | ||||||||||||||||
IRC analysis
| Approach trajectory of endo product formation | IRC analysis of Endo TS |

|

|
| Approach trajectory of Exo product formation | IRC analysis of Exo TS |
|

|
| Approach trajectory of cheletropic product formation | IRC analysis of Cheletropic TS |
|

|
Energy Barrier Calculation
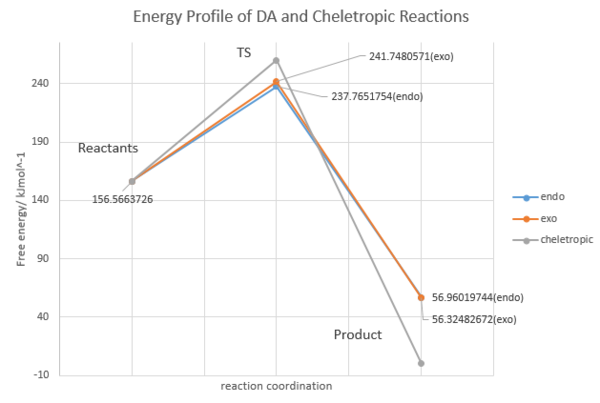
| Components | Energy/Hatress | Energy/kJmol-1 |
|---|---|---|
| SO2 | -0.118614 | -311.42092 |
| xylylene | 0.178247 | 467.9872926 |
| Sum of Reactants energy | 0.059633 | 156.5663726 |
| endo TS | 0.09056 | 237.7651754 |
| endo product | 0.021695 | 56.96019744 |
| exo TS | 0.092077 | 241.7480571 |
| exo product | 0.021453 | 56.32482672 |
| cheletropic TS | 0.099061 | 260.0845411 |
| cheletropic product | -0.000002 | -0.005250998 |
| Components | Activation Barrier/kJmol-1 | Enthalpy Change /kJmol-1 |
|---|---|---|
| Endo | 81.19880277 | -99.60617517 |
| Exo | 85.18168452 | -100.2415459 |
| Cheletropic | 103.5181685 | -156.5716236 |
(Normalise energies to the reactants. It helps when comparing. Also you are quoting extremely high precision Tam10 (talk) 14:33, 7 December 2017 (UTC))
From the reaction energy profile, it can be concluded that: the DA reaction is kinetically favoured and the cheletropic reaction is thermodynamically favoured. Because DA reaction has a lower energy TS but a less stable product (i.e. a small activation barrier and enthalpy change) whereas the cheletropic reaction has a higher energy TS but a more stable product (i.e. large activation barrier and enthalpy change). This can be accounted by the fact that a five- membered ring adduct is more stable than a six membered ring adduct. In the DA reaction, the exo product is slightly more stable than the endo product due to the reason that less steric repulsion exists in exo product.
Ortho-xylylene is highly unstable due to it is non-aromatic and the 2 C=C bonds are in fix cis-geometry. The electron density is very high which makes the xylylene an electron rich (reactive) diene. When a reaction takes place, the 6-carbon-membered ring becomes aromatic and 1 double bond is broken with the formation of 2 less reactive single bonds in return. The reaction is thermodynamically favoured as the products are more stable than the reactants.
(I would have thought the delocalisation would reduce the electron density compared to butadiene. But yes, in comparison to aromatic product it is very reactive Tam10 (talk) 14:33, 7 December 2017 (UTC))
There are two sites on the o-xylylene that can allow pericyclic reaction happen, which site is more favoured ?

| Optimisation of EXO TS | Optimisation of ENDO TS | ||||
| Components | Energy/Hatress | Energy/kJmol-1 |
|---|---|---|
| SO2 | -0.118608 | -311.405327 |
| Xylylene | 0.178935 | 469.793878 |
| Reactants energy | 0.060327 | 158.388550 |
| ExoTS | 0.105053 | 275.816673 |
| EndoTS | 0.102069 | 267.98218 |
| Exo product | 0.067304 | 176.7066655 |
| Endo Product | 0.065611 | 172.2616936 |
| Exo | Endo | |
|---|---|---|
| Activation energy (kJ/mol) | 117.428123 | 109.59363 |
| enthalpy change (kJ/mol) | 18.3181155 | 13.8731436 |
Looking at the animation of IRC of the previous reaction (Table 9), when the cheletropic reaction takes place, the lone pair on the S of the SO2 need to approach to the pi system of the diene linearly. While when DA reaction happens, the SO2 need to approach to the pi system with the electron in the p orbital of S at a skew angle. This means the ability of the diene to rotate their double bonds are crucial in the reaction. There are 2 sites in the o-xylylene that can allow pericyclic reactions happen. However, the orbitals of the inner site diene are restricted to rotate and the inner site is sterically more hindered than the outer site diene for SO2 to approach. Also, the product of the pericyclic reaction of the inner site diene is non-aromatic (pi system not conjugating and non-planar). Thus, the reaction of inner site diene would be highly unfavourable than the outer site diene.
(Good work. Your data also shows that the reaction is predicted to be endothermic Tam10 (talk) 14:33, 7 December 2017 (UTC))
Conclusion
In this exercise, the energies of the 3 pericyclic reactions that have been studied using Gaussian calculation and viewed by Gaussview, are matching with the theoretical predictions. A reaction with a lower activation energy is kinetically favourable and a reaction with a low value of change of enthalpy is thermodynamically favourable.
There are different advantages of the optimisation methods that have been used to find the optimised geometry of reactant, TS and product. It is preferred to draw a correct TS then optimised using Gaussian if the geometry of the TS is known in details since it is a cheaper and faster way. This can be used to confirm the TS. If the TS of a reaction is unknown, then the TS can be found by drawing out an optimised product and breaking the bonds which would be formed during the reaction. This is a slower process. Meanwhile, optimised at different levels gives slightly different results. B3LYP gives a more accurate geometry then PM6, but it takes a longer time to achieve the desired result. When confirming the geometry of a TS, a frequency calculation was run. Only when one negative imaginary frequency value is shown that the TS has the correct geometry. Because a TS locates at a saddle point in a potential energy landscape, where the second derivative (the curvature) only has one negative value (a maximum) in 3D.
In conclusion, Gaussian calculation using a computer provides a precise way to find the energy profile of a reaction and to determine the kinetically / thermodynamically favourable products.