Rep:Mod:Yunzhang Module3 Writeup3
Exercise: The Diels Alder Cycloaddition
In the Diels Alder reactions(6п electrons; Hueckel transition state; suprafacial; heat), the dieneophile π orbitals is involved in forming a new σ bonds with the diene π orbitals. Depends on the number of π electrons involved in this process, the reaction happens in either allowed or forbidden stereospecific fashion.
The Diels-Alder reaction between ethylene and butadiene
In this exercise, the HOMO/LUMO of cis-butadiene interacts with the π/ π* of the ethylene to form two new bonding and anti-bonding MOs([4s + 2s]), and if the HOMO/LUMO of cis-butadiene interacts with the π*/π of ethylene, the reaction is allowed. This interaction can only happen when these orbitals have the same symmetry and overlap siginificantly, otherwise, the reaction is forbbidden.
Exercise
The AM1 semi-empirical molecular orbital method are used for these calculations at the beginning.
i) Sketch cis butadiene in GaussView -> Calculate: Gaussian -> Job type: opt_freq -> Method: AM1 semi-empirical -> additional keywords: ‘pop=full’ -> Submit.
| Jmol of cis butadiene | Results summary | |||
|---|---|---|---|---|
|
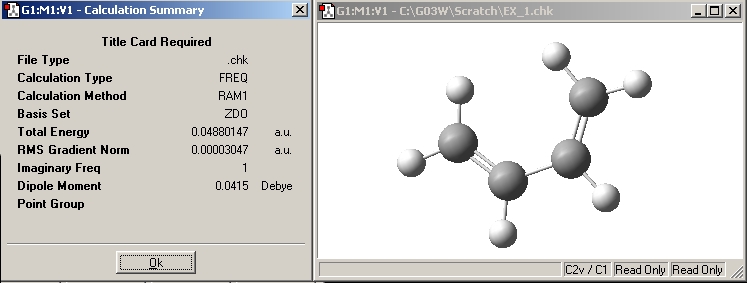 |
Plot the HOMO and LUMO of cis butadiene and determine its symmetry:
| / | HOMO | LUMO |
|---|---|---|
| Cis-butadiene | 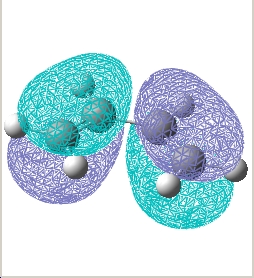 E= -0.343 a.u. |
 E= 0.017 a.u. |
| Symmetry w.r.t plane | Asymmetric | Symmetric |
The above procedures have been repeated for ethylene:
| Jmol of ethylene | Results summary | |||
|---|---|---|---|---|
|
 |
Plot the HOMO and LUMO of ethylene and determine its symmetry:
| / | HOMO | LUMO |
|---|---|---|
| Ethylene | 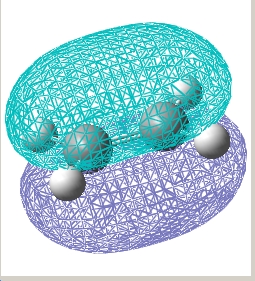 E= -0.387 a.u. |
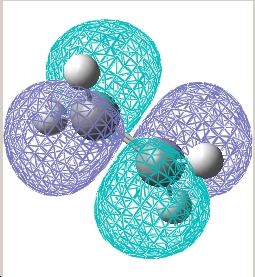 E= 0.052 a.u. |
| Symmetry w.r.t plane | Symmetric | Asymmetric |
ii) Computate the Transition State geometry and examine the nature of the reaction path for the prototype reaction.
Based on the optimisied structures in i) -> draw a guess transition state structure(the guessed interfragment distance between the chain end Cs= 2.1A) -> Calculate: Gaussian -> Job type: opt+freq; optimise to a: TS(Berny); calculate force constants: once -> Method: AM1 semi-empirical -> additonal keywords ‘opt=noeigen’.
The optimization was successfully carried out as the output gives only one imaginary vibraitonal frequency.
| Jmol of Transition State geometry | Results summary | Imaginary frequency | |||
|---|---|---|---|---|---|
|
 |
 -955.785cm-1 |
As can be seen the transition structure has an envelope-like shape, since the overlap between the ethylene π/π* orbitals and the HOMO/LUMO of butadiene can be maximized.
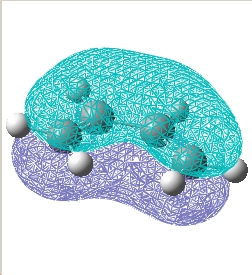
Plot the HOMO and LUMO of transition structure and determine its symmetry:
The plane is defined by the horizental middle plane between the cis butadiene and the ethylene.
| / | HOMO | LUMO |
|---|---|---|
| Transition state | 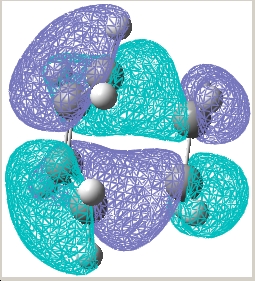 E= -0.323 a.u. |
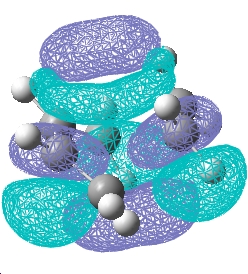 E= 0.023 a.u. |
| Symmetry w.r.t the plane | Asymmetric | Symmetric |
The reaction is allowed in a concerted stereospecific fashion a result of the asymmetric HOMO of cis-butadiene overlaps with the asymmetric LUMO of ethylene; the symmetric LUMO of cis-butadiene overlaps with the symmetric LUMO of ethylene; these HOMO-LUMO pair interactions forms an asymmetric HOMO of the resulting adduct consisting of two new σ bonds.
(iii) Study the regioselectivity of the Diels Alder Reaction between cyclohexa-1,3-diene and maleic anhydride.
As the reaction is controlled kinetically, the cyclohexa-1,3-diene undergoes facile reaction with maleic anhydride to give the endo adduct(transition state energy).
Sketch the endo and exo products in Gaussview -> Calculate: Gaussian -> Job list: opt+freq; once -> Method: HF/3-21G -> Submit. Then, beased on the optimized geometries, the two conformers are optimized futher at B3LYP/6-31G(d) level of theory.
Results for Endo and Exo products:
| Jmol of Endo product | Results summary under HF/3-21G | Results summary under B3LYP/6-31G(d) | Geometric information(HF/3-21G) | Geometric information(B3LYP/6-31G(d)) | |||
|---|---|---|---|---|---|---|---|
|
 |
 |
 |
 |
| Jmol of Exo product | Results summary under HF/3-21G | Results summary under B3LYP/6-31G(d) | Geometric information(HF/3-21G) | Geometric information(B3LYP/6-31G(d)) | |||
|---|---|---|---|---|---|---|---|
|
 |
 |
 |
 |
From the results obtained above, the endo product is lower in energy than the exo one and therefore endo is more favoured, the reason for this is because when the dieneophile is substituted, the π orbitals in substituents can interact with the new double bond which forms in the product, this interaction stabilises the regiochemistry of the endo form more than that of the exo form(so-called the secondary orbital overlap effect), and this effect determines which transition state conformer is more favoured.
The optimisation was carried out by using the Hessian method based on a guessed transition structure(intermolecular bond length of the chain end carbons= 2.1A), but in order to get a reasonable guessed transition struture, both cyclohexa-1,3-diene and maleic anhydride were optimized first and then combined together to give the exo and endo transition structures.
Results for Endo and Exo tansition states:
| Jmol of Endo transition state | Results summary | Geometric information | Imaginary frequency | |||
|---|---|---|---|---|---|---|
|
 |
 |
 -805.614cm-1 |
| Jmol of Exo transition state | Results summary | Geometric information | Imaginary frequency | |||
|---|---|---|---|---|---|---|
|
 |
 |
 -811.515cm-1 |
Results for the HOMO/LUMO for the Exo and Endo Transition state:
| / | HOMO | LUMO |
|---|---|---|
| Endo | 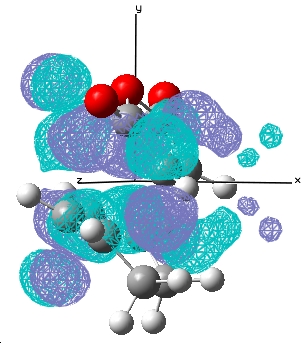 E= 0.028 a.u. |
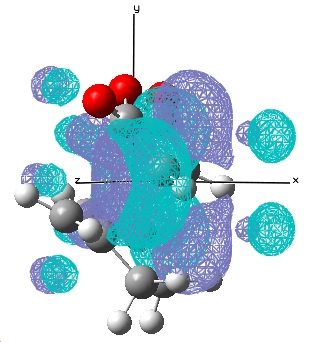 E= -0.020 a.u. |
| / | HOMO | LUMO |
|---|---|---|
| Exo | 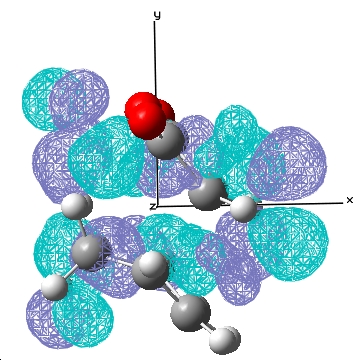 E= 0.033 a.u. |
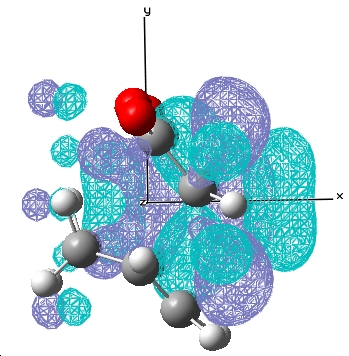 E= -0.020 a.u. |
The typical sp3 and sp2 C-C bondlengths are 1.54Å and 1.34Å respectively, the double bond in cyclohexa-1,3-diene at the transition state is 1.397Å which is somewhere in between a double and single carbon carbon bond. The van der Waal radius of carbon is 1.702Å and the bond forming distances for the endo and exo transition states are 2.16Å, 2.17Å respectively, which indicates the orbitals do not overlap significantly and hence gives rise to a transition state in this case.
The formation of the two bonds is synchronous and no similarity has been senn when compare with the lowest positive frequency.
For the reaction of cyclohexa-1,3-diene with maleic anhydride, the exo product is more strained because the bridging group and the -C=O-CO-C=O- fragment are pointing in the same direction whereas in the endo product, they are pointing away from each other. There is only one major nodal plane has been observed which is in between the HOMO between the -C=O-CO-C=O- fragment(x-axis, in this case).
