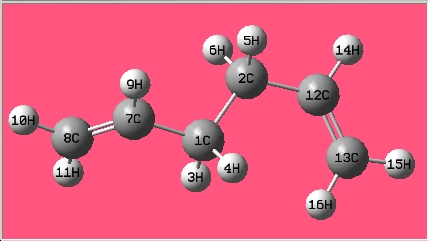
Rep:Mod:Yunzhang Module3 Writeup1
Module3, Experiment 3: The Transition State
In this experiment, the transition state structures in larger molecules for Cope rearrangement and Diels Alder cycloaddition reactions are studies using molecular orbital-based computaional methods which solve the Schrodinger equation numerically and locate the transition state structures based on the local shape of a potential energy surface. It also gives us information about the shapes of the transition structures, the pathways in which the reaction undergoes and how big the barrier heights are.
The Cope Rearrangement Tutorial
Chemical reactivity of the Cope rearrangement of 1,5-hexadiene
Objectives:
locate the low-energy minima and transition structures on the C6H10 potential energy surface and determine the preferred reaction mechanism.
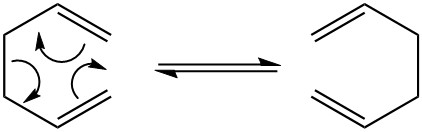
The [3,3]-sigmatropic shift rearrangement occurs in a concerted fashion(6 electrons; 4n+2; heat)via either a "chair" or a "boat" transition structure, with the "boat" transition structure lying several kcal/mol higher in energy. The B3LYP/6-31G* optimisation was carried by using Gaussian and see how it matchees with this result.
| Cope rearrangement | Chair Transition State | Boat Transition State |
|---|---|---|
 |
 |
 |
Optimizing the Reactants and Products
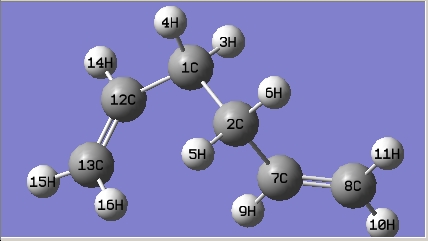
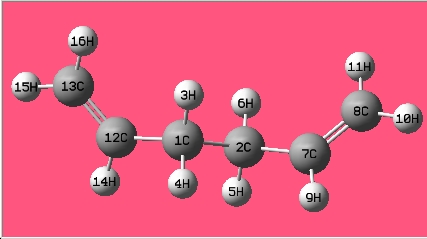
(a) Open GaussView -> in the Molecule window: draw 1,5-hexadiene with an "anti" linkage for the central four C atoms -> click "Clean" under the "Edit" menu.
"Calculate" -> "Gaussian" -> "Job type": optimization -> "Method": Hartree-Fock -> "Basis set": 3-21G -> "Link 0": %mem=250MB -> Submit -> save as "yun_react_anti" -> after job has finished, open the file -> Select "Yes" -> Files of type: choose *.chk -> Open "yun_react_anti" in the C:\Windows\G03\Scratch folder -> "Result": Summary -> "Edit": Symmetrize.
| 1,5-hexadiene with an "anti" linkage
for the central four C atoms |
Results Summary | |||
|---|---|---|---|---|
|
 |
The structure is the same one as Anti 3 in Appendix 1
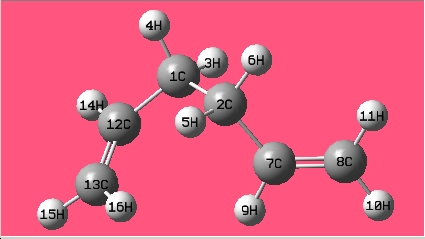
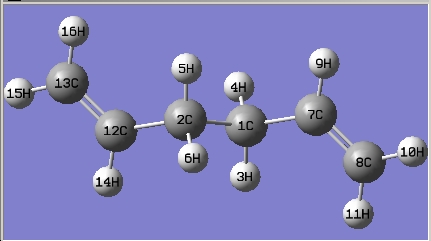
(b) Draw 1,5-hexadiene with a "gauche" linkage for the central four C atoms and optimize the structure at the HF/3-21G level of theory.
Would you expect this structure to have a lower or a higher energy than the anti structure you have just optimized?
Both gauche and anti conformers are very similar in enenrgy; if the electrostatic effect(eg. gauche effect) overweighs the steric effect, the gauche conformers are preferred and vice versa.
| 1,5-hexadiene with a "gauche" linkage
for the central four C atoms |
Results Summary | |||
|---|---|---|---|---|
|
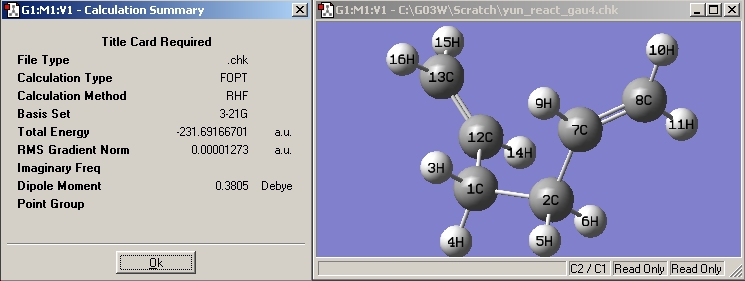 |
The structure is the same one as Gauche 4 in Appendix 1
(c)-(e) Based on the results above, predict the lowest energy conformation of 1,5-hexadiene and test out your hypothesis by drawing the structure and optimizing it.
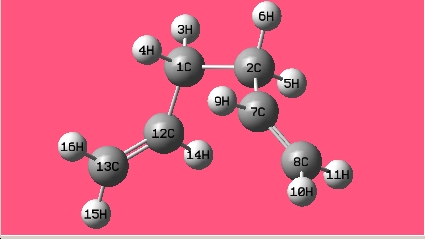
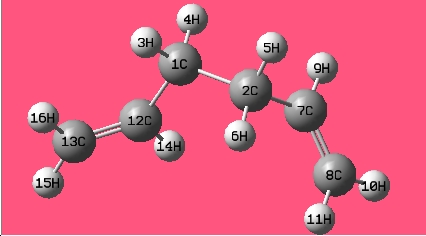
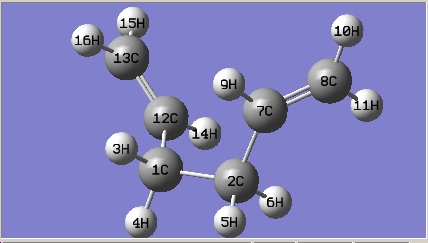
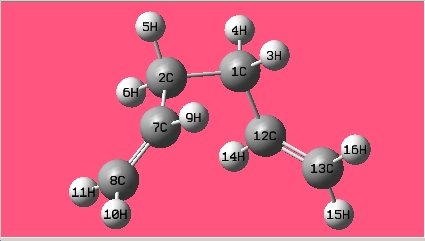
The gauche conformer for 1,5-hexadiene is more stable than that of the anti conformer from thre optimisation above; in the hypothesis test, all the gauche and anti conformers have been optimised at HF/3-21G level of theory. The names of the structure optimized are given by the matching structure in [Appendix 1].
Hypothesis test:
| Gauche conformer | Jmol | Results Summary | |||
|---|---|---|---|---|---|
| Gauche1 |
|
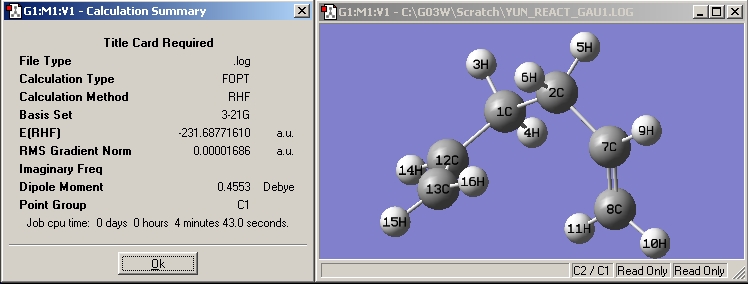 | |||
| Gauche2 |
|
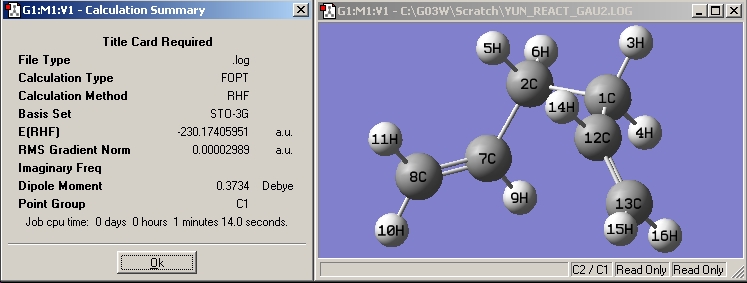 | |||
| Gauche3 |
|
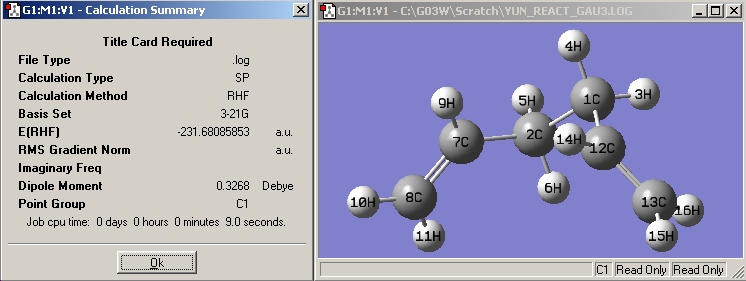 | |||
| Gauche5 |
|
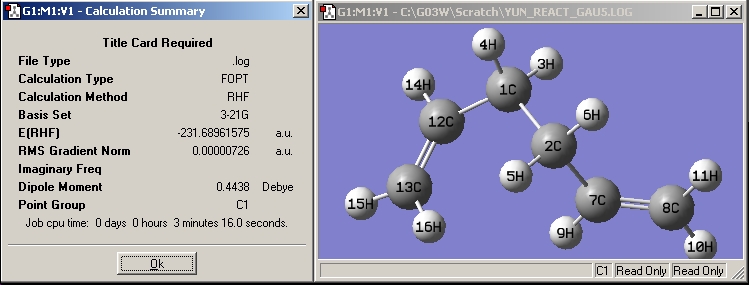 | |||
| Gauche6 |
|
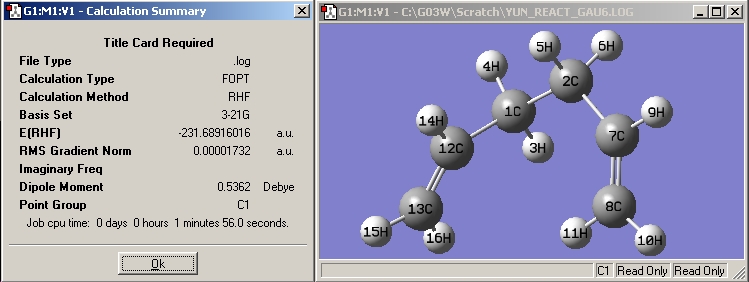 |
| Anti conformer | Jmol | Results Summary | |||
|---|---|---|---|---|---|
| Anti1 |
|
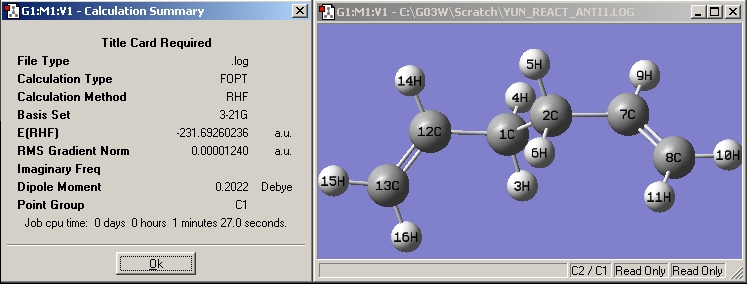 | |||
| Anti2 |
|
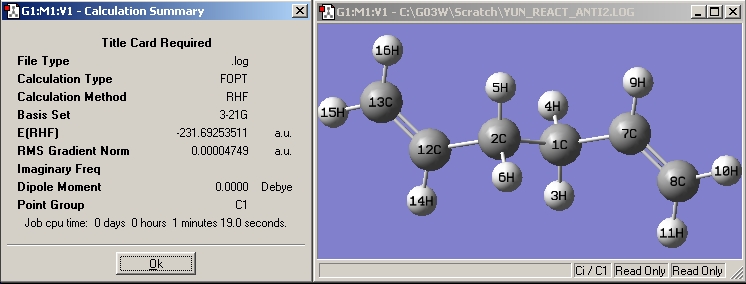 | |||
| Anti4 |
|
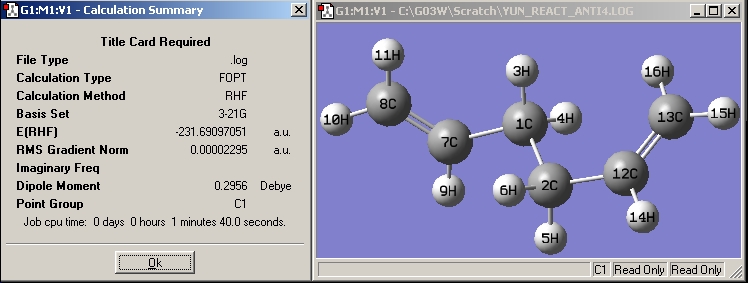 |
The energy obtained for each conformer is well matched with the ones shown in [Appendix 1] which conforms the optimisation has been carried out correctly.
(f) The structures are reoptimized at the B3LYP/6-31G(d)level:
| Gauche conformer | Jmol | Results Summary | |||
|---|---|---|---|---|---|
| Gauche1 |
|
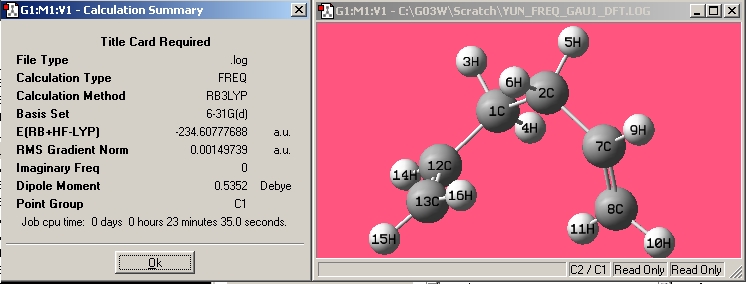 | |||
| Gauche2 |
|
 | |||
| Gauche3 |
|
 | |||
| Gauche4 |
|
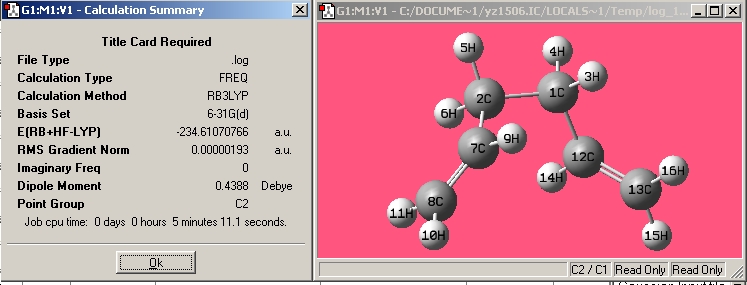 | |||
| Gauche5 |
|
 | |||
| Gauche6 |
|
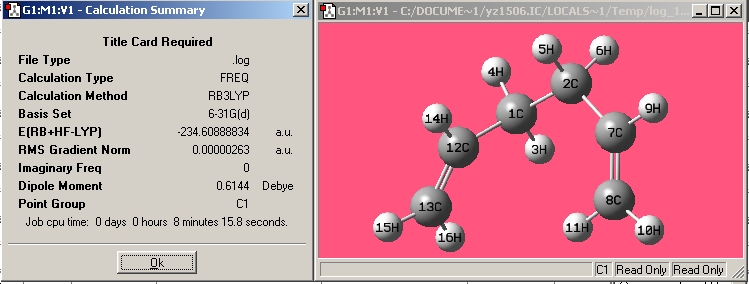 |
| Anti conformer | Jmol | Results Summary | |||
|---|---|---|---|---|---|
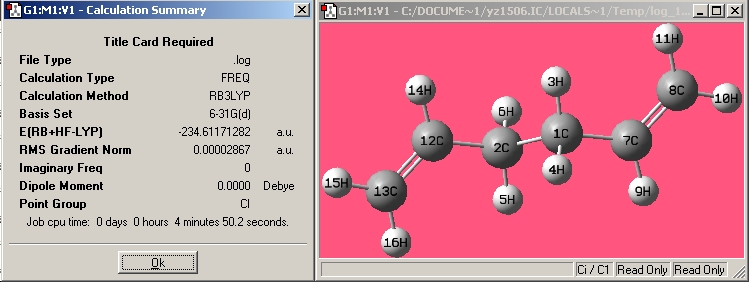
| Anti1 |
|
 | |||
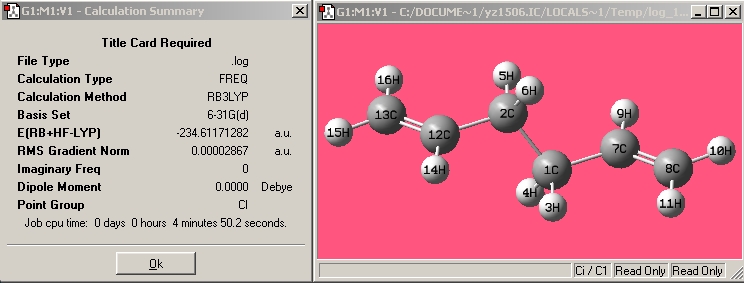
| Anti2 |
|
 | |||
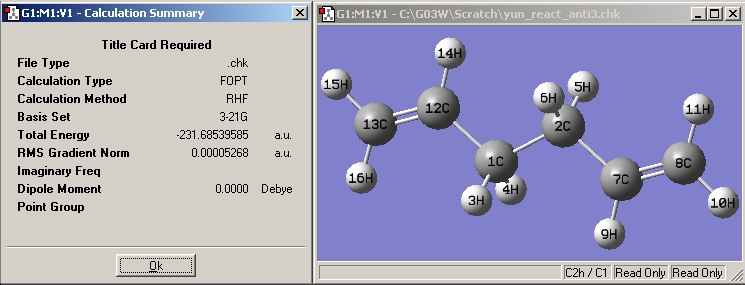
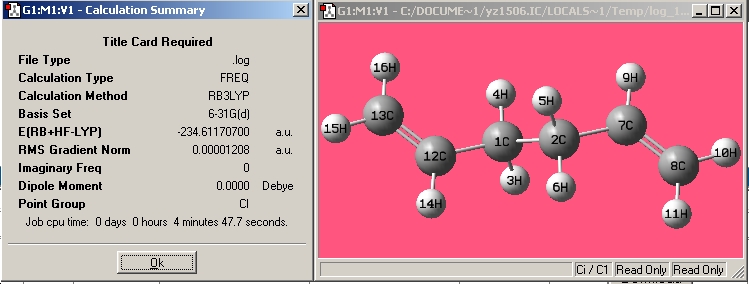
| Anti3 |
|
 | |||
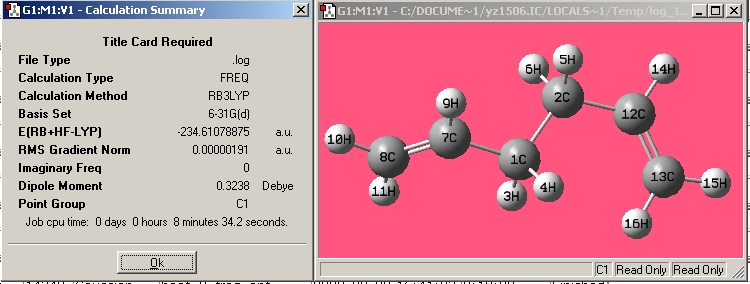
| Anti4 |
|
 |
Compare the final structures from the HF/3-21G calculation with that at the higher level of theory. How much does the overall geometry change?
There is no significant change in geometry and all the point group stays the same for for all the conformers when a higher level of optimization is carried out. However the energy is minimized further when the B3LYP/6-31G(d) level of theory is applied. Geometric information under B3LYP/6-31G and B3LYP/6-31G(d) methods:
(g) Open the optimized B3LYP/6-31G(d) structure -> Calculate: Gussian -> Job type: Frequency -> Method: DFT; B3LYP/6-31G(d) -> link 0: yun_freq_gau1_DFT.chk -> save -> run -> Once the job has finished, open yun_freq_gau1_DFT.log -> Results menu: Vibrations -> Check there are only real frequencies -> Spectrum.
There is no imaginary vibrational frequencies for all the structures as shown in the B3LYP/6-31G(d) result summary table, this means that a minimum energy is obtained for all the structures.
On Results menu: View File -> find Thermochemistry -> find Vibrational temperatures: a list of energies -> Make a note of (i) the sum of electronic and zero-point energies(E = Eelec + ZPE); (ii) the sum of electronic and thermal energies(E = E + Evib + Erot + Etrans); (iii) the sum of electronic and thermal enthalpies(H = E + RT); (iv) the sum of electronic and thermal free energies(G = H - TS).
Results:
| Name of structure | Energy data |
|---|---|
| Gauche1 |  |
| Gauche2 |  |
| Gauche3 |  |
| Gauche4 |  |
| Gauche5 |  |
| Gauche6 |  |
| Anti1 |  |
| Anti2 |  |
| Anti3 |  |
| Anti4 |  |