Rep:Mod:Yts15
Molecular Reaction Dynamics: Applications to Triatomic systems
Introduction
Reactivity of triatomic system, H + H2 and F-H-H system are studies through calculating the Molecular dynamics trajectories. Hence, transition state position can be located. Activation energy of the reaction is also determined from the potential energy vs time graph. In addition, reaction is illustrated by Polanyi's emperical rules. Particularly, H and HF is an endothermic reaction. Hence, it has a late barrier and vibrational energy will promote the reaction more according to Polanyi's emperical rule.
Reaction 1: H + H2 system
The initial condition is set to be r1 = 0.74 Å., r2 = 2.30 Å and the momenta p1 = 0 Ns p2 = -2.7 Ns.
In this case AB is the molecule with a bond distance = 0.74 Å and C is the atom that collides with it. Hence, r1 = rBC
Dynamics from the transition state region
At a minimum and at a transition structure, total gradient of the potential energy surface have zero value since they are stationary points. The two states can be distinguished by second derivative . Since the transition state is defined as the maximum on the minimum energy path linking reactants and the products, and hence it lies on the maximum point on the curve. The second derivative of transition structures will be negative. While for minima, the second derivative will be positive.
For transition state,
For minima,
Nf710 (talk) 15:42, 2 June 2017 (BST) this is only in the basis of normal modes where one of them is the reaction coordinate.
Trajectories from r1 = r2: locating the transition state
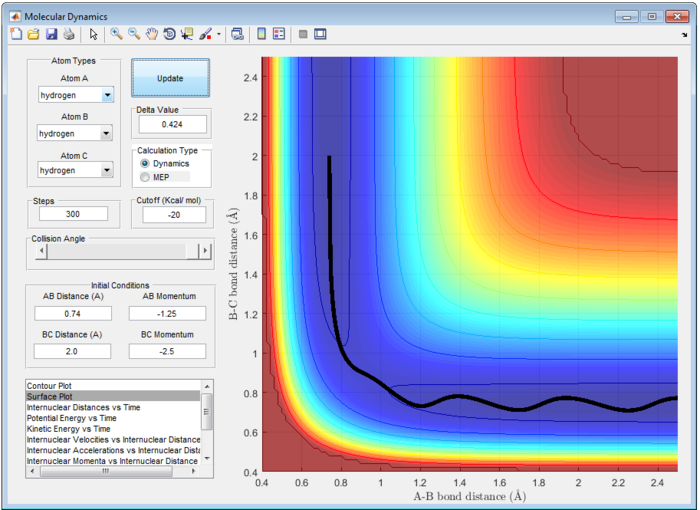
From Figure 1, it shows the transition state position (rts) at 0.9079 Å. At this position, the internuclear distance between A-B and B-C remain constant and equal to each other. Atom A and C stays stationary and are not likely to vibrate symmetrically at this position. Hence, gradient dV/dr at transition state is zero which showing the force between each atoms are zero.
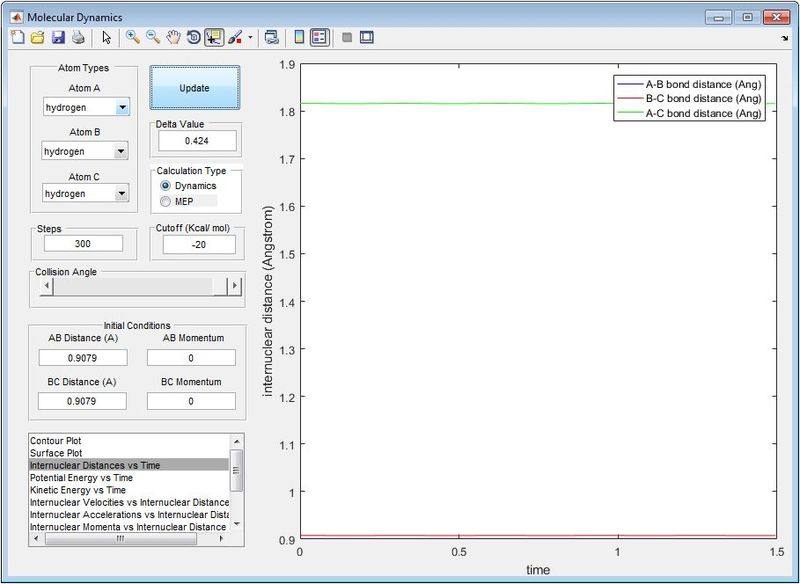
Trajectories from r1 = rts+δ, r2 = rts
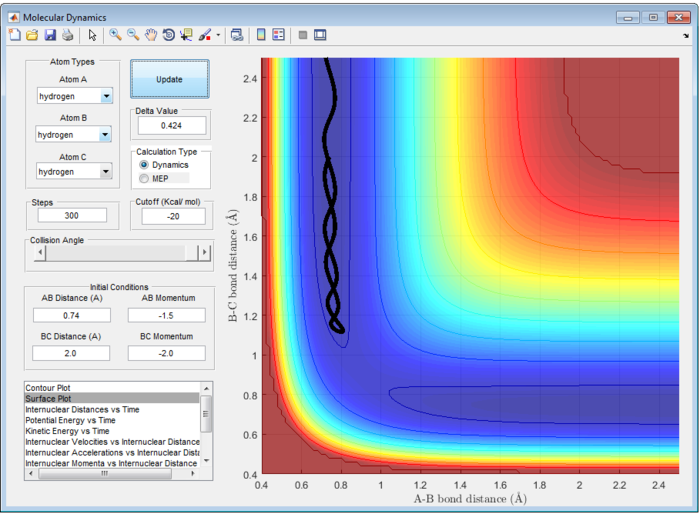
Minimum Energy Pathway (MEP) resets velocity to be zero in each step. It does not consider the realistic motion of atoms such as vibration and follows minima of the ‘valley’ towards the transition state [1]. Hence, MEP shows a straight line trajectory from Figure 2. In contrast, vibrational energy is taken into account in dynamics calculations,. The trajectory from Dynamic calculation follows an oscillation path in Figure 3.
 |
 |
Reactive and unreactive trajectories
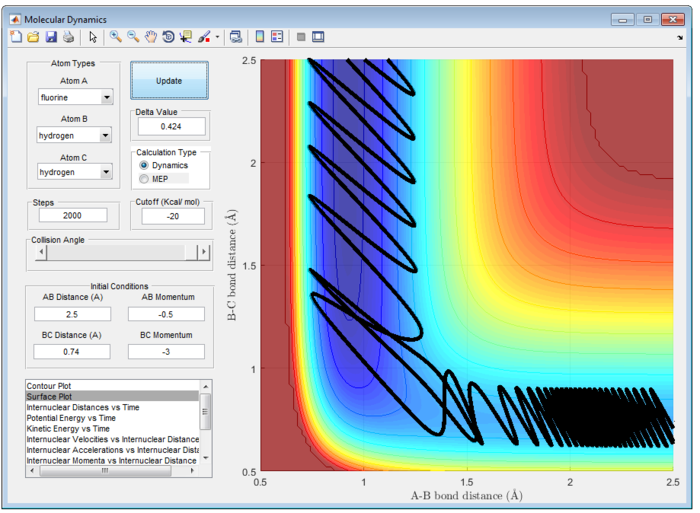
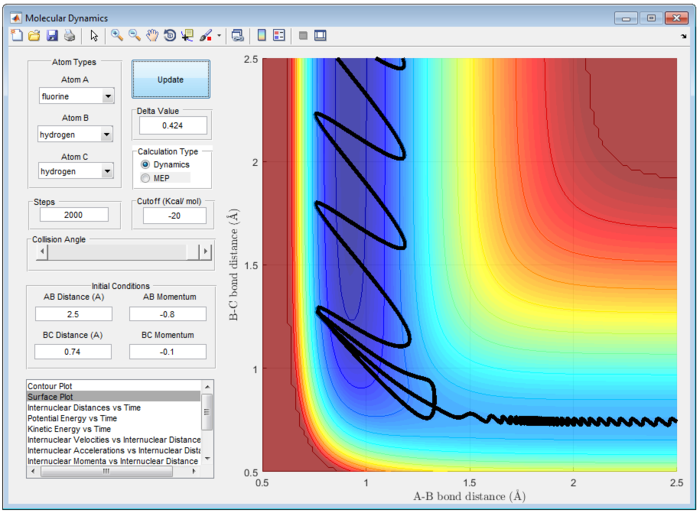
It is important to note that the reaction is only reactive when r1 = 0.74 and r2 = 2.0 and p1 = -2.5 Ns and -1.5<p2<-0.8 Ns.
- For the initial positions r1 = 0.74 and r2 = 2.0, run trajectories with the following momenta combination:
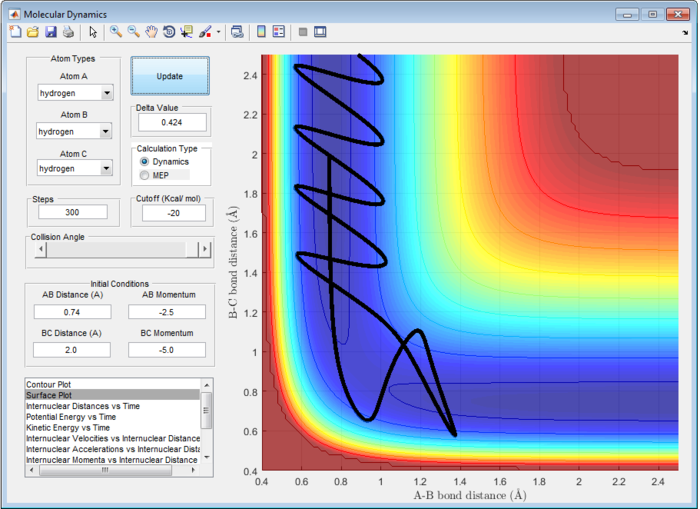
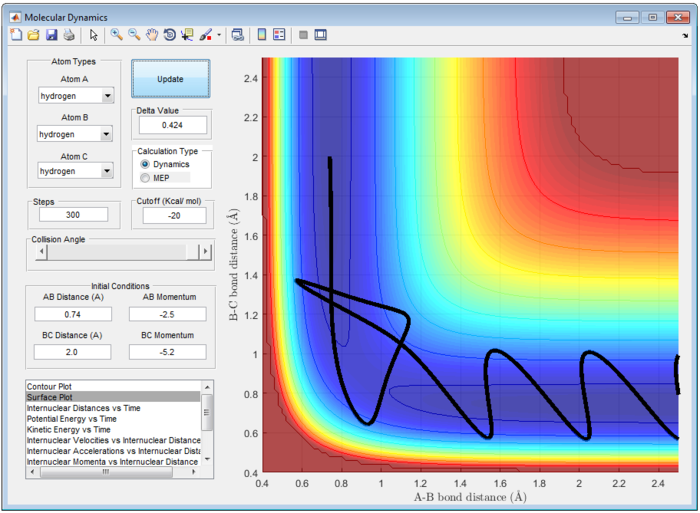
The Main Assumption of Transition State Theory (TST) [2] is the motion of atoms follow classical mechanics since the mass of electrons are negligible compared to the atom. Quantum effect such as quantum tunneling and quantised vibration are not considered in this assumption. Hence, reaction can only pass through the transition state once from reactant to product or vice versa. However, from figure 7 and 8, reaction does not follow the classical transition state theory and reaction recrosses transition state.
According to TST, TST rate is proportional to the flux of classical reaction trajectory. It can be calculated from Maxwell-Boltzman weighting at a given temperature or delta function weighting to count trajectories of a given total energy.
As a result, TST only work at low temperature. At high temperature, reaction might not pass through saddle point, namely the transition state and rate of reaction will affect by other factors as well. Hence, experimental values will be lower than the one predicted by TST since chemical environments and vibration will affect reactivity of reactants.
Nf710 (talk) 15:56, 2 June 2017 (BST) Good understanding could have backed it up with an equation.
Reaction 2: F-H-H system
In this system, there are two possible reactions.
F atom can react with H2 molecule to give HF molecule and H atom.
Alternatively, H atom can react with HF molecule to give H2 molecule and F atom.
PES Insepction
F + H2 reaction
Firstly, F atom react with H2 molecule to give HF molecule and H atom. Due to the large electronegativity difference between and F and H, ionic bond is formed between H and F. While H-H form covalent bond. Hence, the bond enthalpy of H-F is stronger than that of H-H (as ionic bond is much stronger than that of covalent bond). Therefore, energy required to break the H-H bond is less than the energy released when H-F is formed. Hence, (enthalpy equation). The reaction is exothermic.
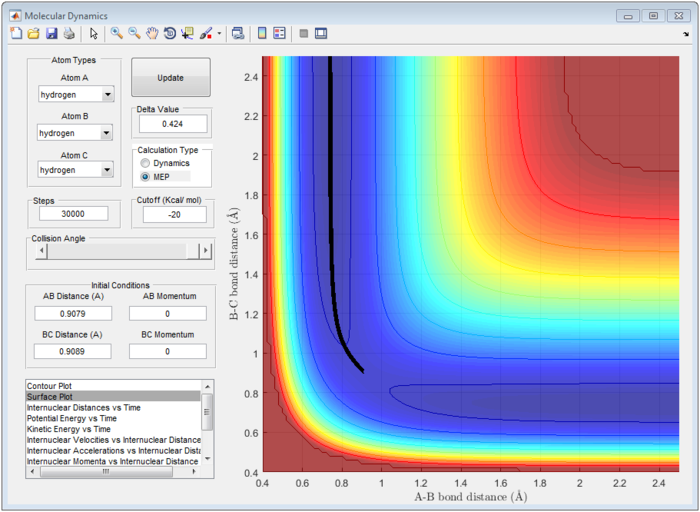
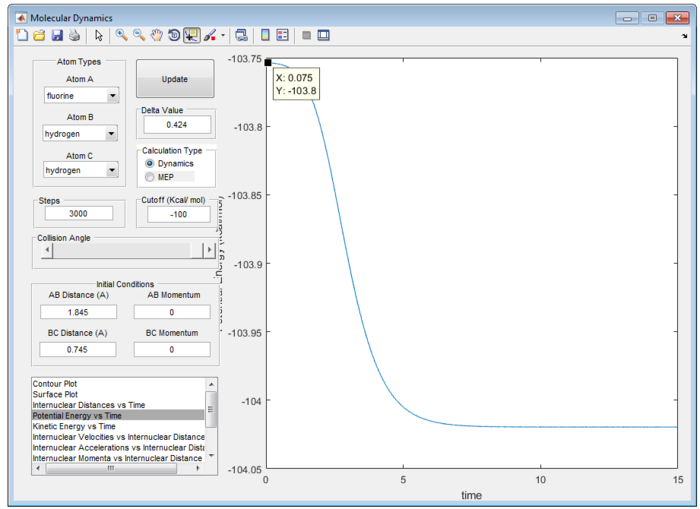
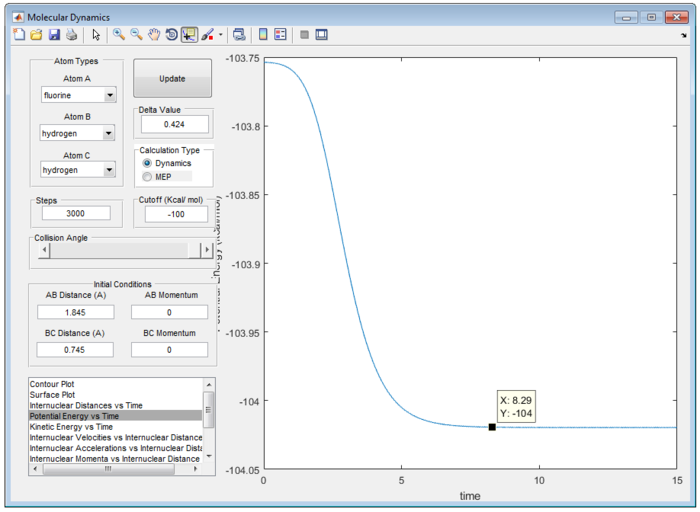
Therefore, the transition state of the reaction is found at rFH equal to 1.810 Å and rHH equal to 0.745 Å as shown in figure 9.

According to Hammond’s postulate, in an exothermic reaction, energy of the transition state is closer in energy to that of reactant than that of intermediate or the product.[3] Activation energy of the reaction can be obtained from the difference between transition state and the product as follow.
Potential Energy for the Transition State = -103.8 kcal/mol
Potential Energy for the H-F / H = -104.0 kcal/mol
Therefore, the activation energy for F + H2 reaction is +0.2 kcal/mol as shown in figure 10(a) and (b).
 |
 |
H + HF reaction
Secondly, H atom react with HF to give HF molecule and H2 molecule. Since H-F bond enthalpy is much stronger than that of H-H bond, energy required to break the H-F bond is much larger than that of H-H released. Hence, the reaction is endothermic.
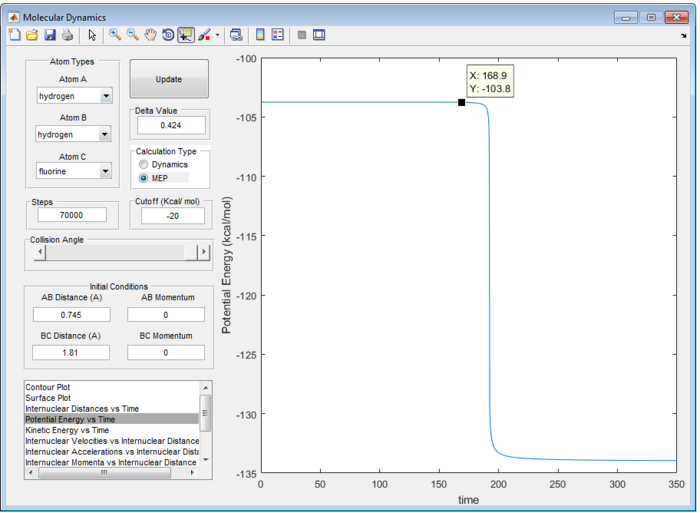
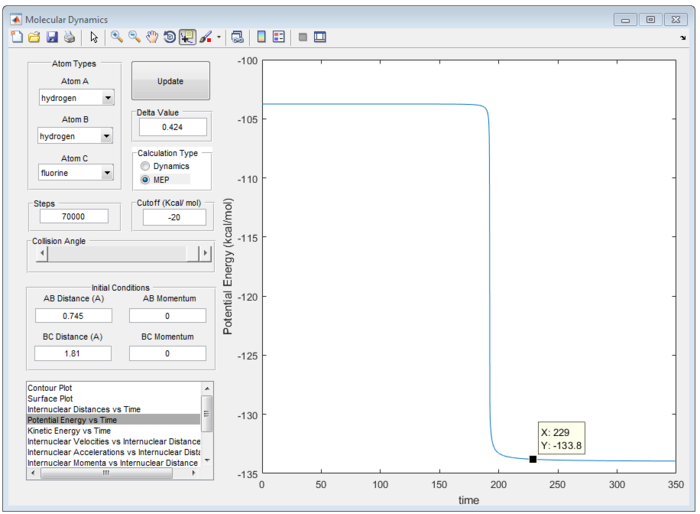
Therefore, the transition state of the reaction is found at rFH equal to 1.810 Å and rHH equal to 0.745 Å as shown in figure 9.

In an endothermic reaction, according to the Hammond's Postulate, transition state should closely resemble to that of intermediate or the energy [ref]
Potential Energy for the Transition State = -103.8 kcal/mol
Potential Energy for reactant = -133.7 kcal/mol
Therefore, the activation energy for H + HF reaction is +30.1 kcal/mol as shown in Figure 11 (a), (b).
 |
 |
Reaction dynamics
The initial condition for reactive trajectory is rFH = 2.5 Å and rHH=0.745 Å; pAB = -2.5 pBC = 0. Since the reaction is exothermic, high potential energy is converted to kinetic energy and potential energy. At transition state, F atom collide with H-H molecule. Hence, the translational potential energy of F atom is transferred to vibrational energy of H-H. Vibrational energy of H-H is very high and causing the bond to break as demonstrated in figure 12 and 13.
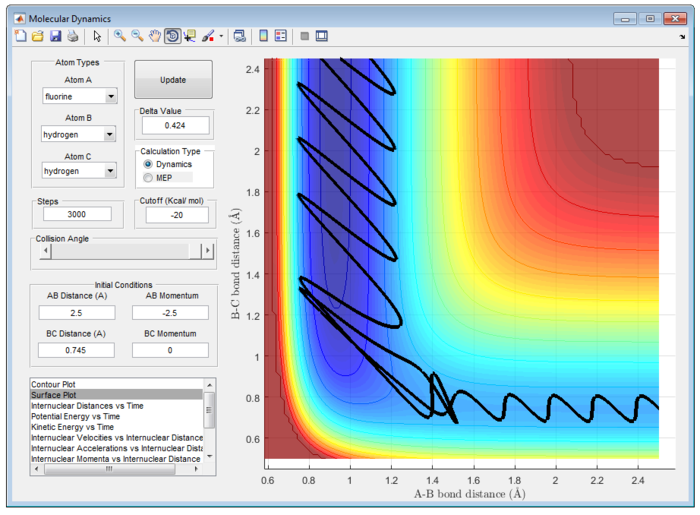 |
 |
In addition, the mechanism of release of reaction energy can be confirmed experimentally.
Thermometer can be used to monitor the temperature increase when molecules are vibrating to the excited state.
Nf710 (talk) 16:02, 2 June 2017 (BST) Calorimetry
From eqaution Q = mcΔT, where Q is the thermal energy released or absorbed, m is mass and ΔT is the change of temperature, energy released of the reaction can be calculated.
Secondly, IR can also be used to confirm the mechanism. Since vibration of H-H, F-F and H-F changes as reaction proceed, the peak is shifted and evolved. Vibrational energies can be calculated by E=hv, where h = planck constant and v = wavenumber.
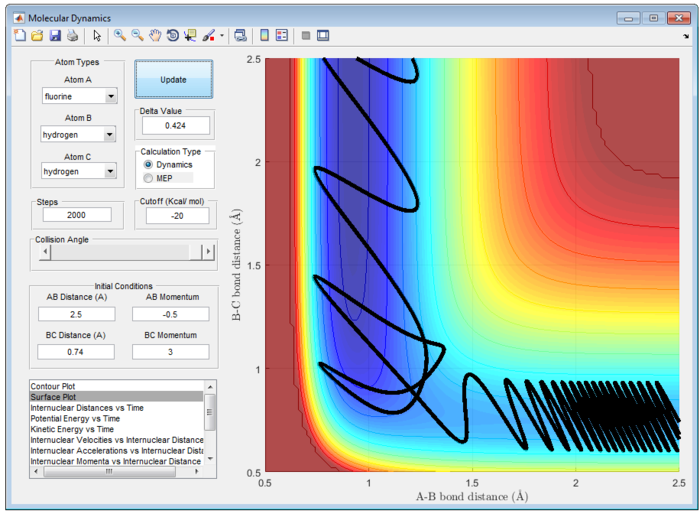
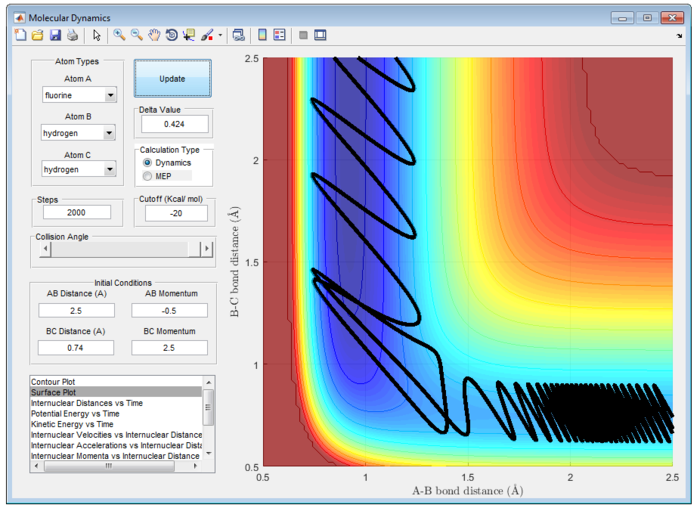
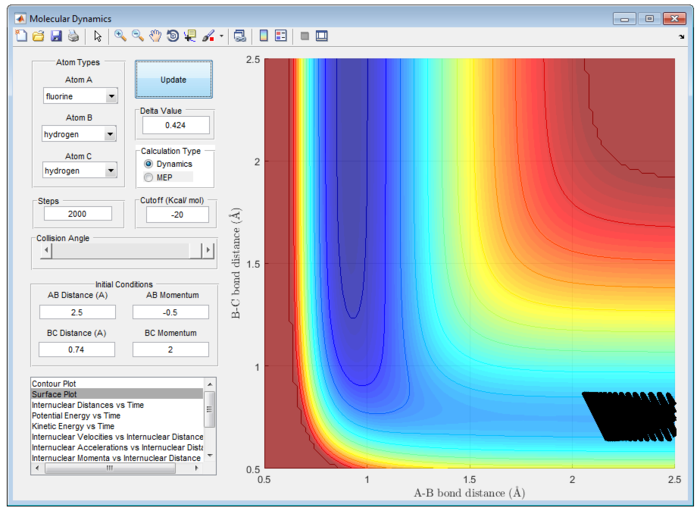
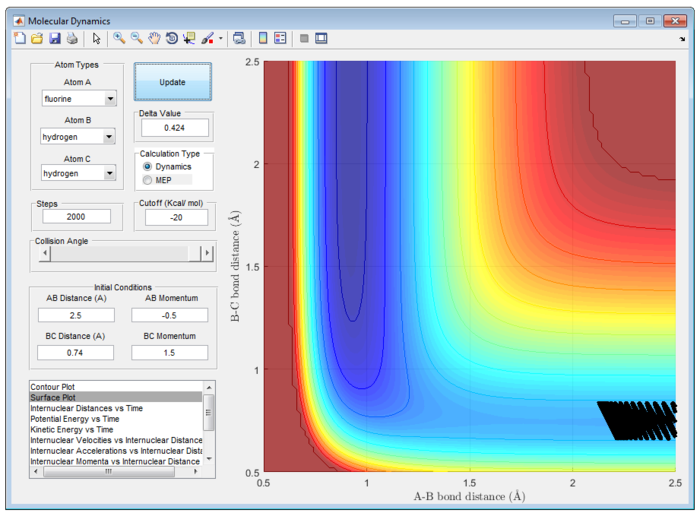
Different combination of pHH/Ns and pFH/Ns in the reaction F + H2 are calculated at table 2 to explain the reactivity.
Table 2 shows the reactivity of F + H2 with various combination of pHH/Ns and pFH/Ns at rFH = 2.5 Å and rHH=0.74 Å.
Polanyi's Emperical Rule illustrate reaction efficiency of the triatomic system. In particular, vibrational excitation of the diatomic reactant enhances reactivity more effectively than translational excitation at high energies, while vice versa at low energies. [4] It is important to note that it is valid only in certain energy ranges divided by a cross-over point. This feature is irrespective of the barrier location.
Since this reaction is exothermic, activation barrier will be smaller and the energy of transition state is more closely to that of energy of reactant, which is earl barrier. Therefore, according to Polanyi's Emperical Rule, translational energy will enhance the reactivity more effectively than vibrational energy. In contrast, for endothermic reaction, the activation energy barrier will be larger and the energy of transition state will be more closely to that of product. A late barrier is formed and hence, vibrational energy will promote the reactivity more effectively than translational energy.
Particularly, H and HF is an endothermic reaction. Hence, it has a late barrier and vibrational energy will promote the reaction more according to Polanyi's emperical rule. Therefore, it is expected that momentum of H-F will be larger than that of H-H atom. This is exemplify in figure 28. However, this may not be an effective reaction. It is observed that there is high input energy at the start of reaction and is only converted to lower vibrational energy at the end of reaction.
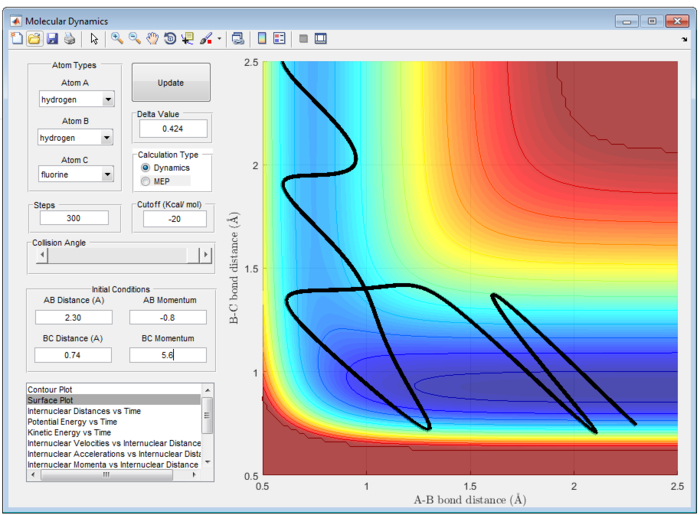
16:06, 2 June 2017 (BST) This is a very nice section you could have shown the endo reaction being unreactive
Conclusion
For H and H2, the transition state position (rts) is located at 0.9079 Å. According to classical Transition State Theory, motion of atoms follow classical mechanics and hence, reaction can only pass through the transition state once from reactant to product or vice versa. However, from figure 7 and 8, reaction does not follow the classical transition state theory and reaction recrosses transition state at high energy.
On the other hand, for the tri-atomic system F-H-H, there are two possible reactions.F atom can react with H2 molecule to give HF molecule and H atom, which is an exothermic reaction. Alternatively, H atom can react with HF molecule to give H2 molecule and F atom which is an endothermic reaction. Activational energy is found to be +0.2 Kcal/mol and +30.1 Kcal/mol respectively. In addition, reaction is illustrated by Polanyi's emperical rules. Particularly, H and HF is an endothermic reaction. Hence, it has a late barrier and vibrational energy will promote the reaction more according to Polanyi's emperical rule.
16:06, 2 June 2017 (BST) Good report
References
- ↑ Computational Theoretical Organic Chemistry; Springer Science Business Media B.V., 1981.
- ↑ Steinfeld, J. I.; Francisco, J. S.; Hase, W. L. Chemical kinetics and dynamics; Prentice Hall: Upper Saddle River, NJ, 1999.
- ↑ Yarnell, A. Chemical & Engineering News Archive 2003, 81 (20), 42.
- ↑ Jiang, B.; Guo, H. The Journal of Chemical Physics 2013, 138 (23), 234104.