Rep:Mod:YYTmod2
Year 3 Computational Lab
Module 2: Bonding (Ab initio and density functional molecular orbital)
Day 1 and Day 2
* BH3 Optimization and Analysis


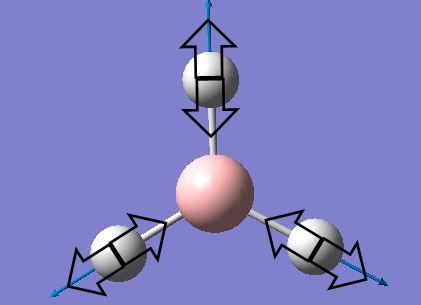
The BH3 molecular model was created in Gaussview and optimised using B3LYP/3-21G. For the optimized result, all B-H bond lengths were 1.19Å, and all H-B-H angles were 120.0o. And the result summary showed that its energy was -26.46226338±0.00381 a.u..(Energy has an error of ~10 kJ/mol, which is 0.00381 Hartrees)
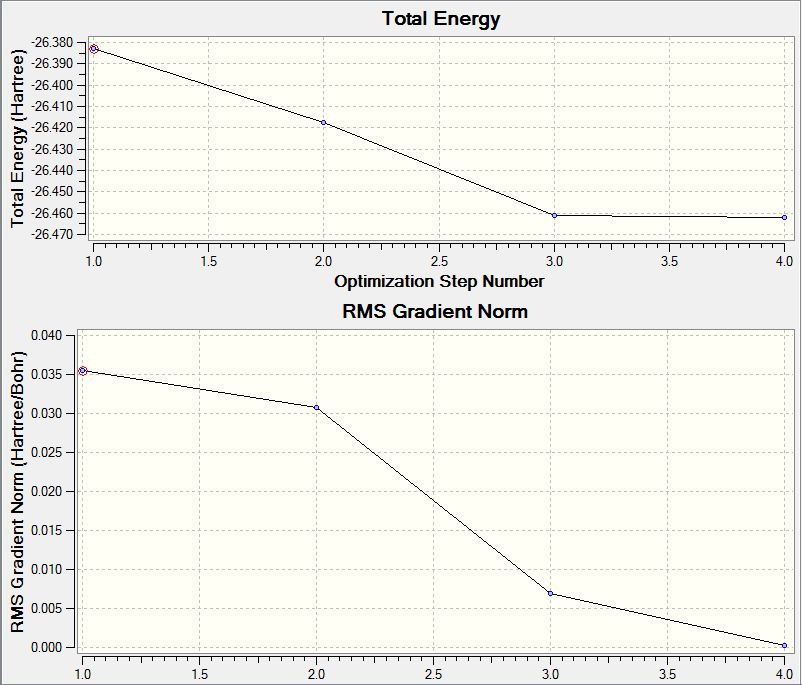
Structure optimization can be achieved by adjusting bond angles and bond lengths. When the distance between two atoms is shorter than the equilibrium one, repulsion occurs to pull the atoms back to the equilibrium position. When the distance between two atoms is longer than the equilibrium one, the attractive force exists. So when all atoms are at equilibrium position, or in an other word, the optimized structure, has lowest energy. The two graphs below were shown as the optimization result. Since the optimized structure has the lowest steric energy, it can be found at the lowest point of the energy/optimization step number graph (the upper one). At this point, the gradient is zero, that is, Δ(Energy)/Δ(Optimization step number)=0. Therefore, in this case, the optimization calculation finished at step 4.0 as the gradient of energy/step number reached zero and the lowest energy structure was obtained.
* TlBr3 Optimisation Using Pseudo-potentials and Larger Basis Sets


TlBr3 molecule was created in Gaussview, and the structure optimization was computed using LanL2DZ basis set after restricting the symmetry of this molecule. The optimised structure was trigonal planar, where all Tl-Br bond lengths were 2.65Å, and all Br-Tl-Br bond angles were 120.0o. In the result summary, the energy was -91.21812851±0.00381 a.u..
* TlBr3 Questions
DFT/B3LYP method was used for ground state optimization, and the basis set was LanL2DZ. Because the energy calculated highly depends on the method used, results for comparison should to be carried out using the same computational method in order to improve accuracy. As mentioned previously, in the energy diagram, both the maximum and the minimum point have zero gradient. To find out whether the structure has the lowest steric energy, second derivative calculation (frequency analysis) is necessary. In this task, energy shown in the summary of frequency calculation was the same as that of the optimization. That is, the optimization did have the lowest energy as expected.
In literature, TlBr3 has a planar triangular geometry with a Tl-Br bond length of 2.512Å. (DOI:10.3891/acta.chem.scand.36a-0125 ) This is similar to the result computed, whose Tl-Br bond lengh was slightly longer but still reasonable.
In some structures, some expected bonds are not shown in Gaussview as the bond lengths are longer than those on the internal list. However, this does not mean these invisible bonds do not exist, especially for inorganic compounds which usually have longer bond lengths than organic complexes.
A chemical bond is an electromagnetic attraction between atoms that holds the atoms in a chemical substance together. This includes strong ionic bonds due to electron transfer and covalent bonds due to bonding electron pair sharing, as well as dipole-dipole interactions such as hydrogen bonding.
* Vibrational Analysis

In the energy diagram, the point with zero gradient can be either maximum or minimum. To confirm this, the second derivative calculation is required. Therefore, the frequency (or vibrational) analysis was used to proof that the optimization had minimum energy. In the summary of result, the total energy was -26.46226338 a.u., which was the same as that of the previous optimization, and the optimized structure was proved to have minimum energy.
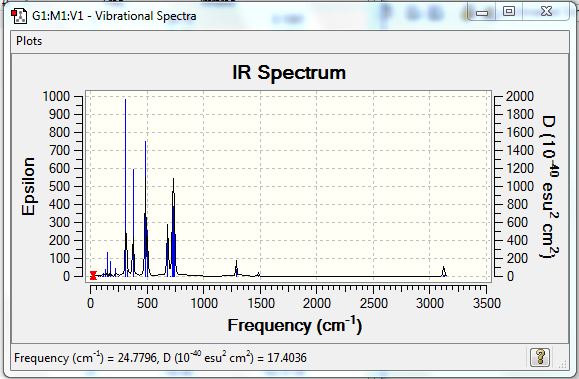
Vibrational results were shown in the table below:
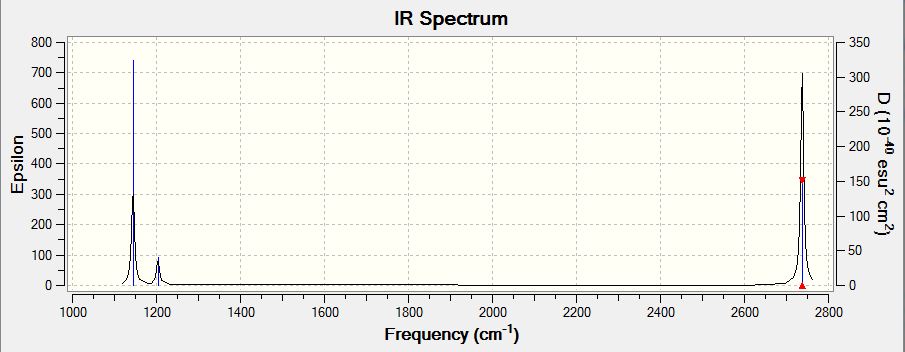
Above is the IR spectrum of BH3 computed by Gaussview. Though there were 6 vibrational modes as mentioned previously, only five of them were shown on the spectrum. That was because vibration 4 was highly symmetrical, and therefore IR inactive.
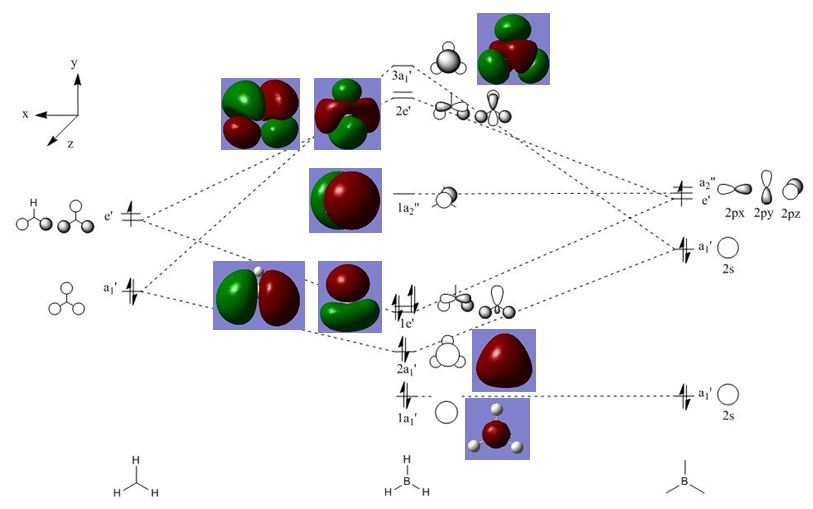
* NBO Analysis
BH3 MO Calculation:DOI:10042/to-9784
Above is the charge representation by colour, where bright green indicates high positivity and bright red refers to high negativity. According to the result, the three hydrogens had a charge of -0.111eV, and the B atoms had +0.332 eV. This could be found in the .log file of the MO calculations. More information about the electronic structure could also be found in this file.
* MO Questions
The MO diagram of BH3 above was drawn using Chemdraw, and the real MOs were computed by Gaussian for comparison. As shown, the shapes of the LCAOs and the real MOs were generally similar. However, LCAOs clearly showed the composition of a specific MO. But for the coloured real MOs, orbitals combined the nearby orbitals which were in the same phase forming larger orbitals. Also, the unoccupied real MOs were more diffuse than the occupied ones. This implies that qualitative MO theory can give a rough idea of the shape and relative energy of molecular orbitals, but further calculations are necessary to obtain more accurate results.
Day 2 and Day 3 : Cis and Trans Isomerism
Cis-isomer LANL2MB Optimization:DOI:10042/to-9791
Cis-isomer LANL2DZ Optimization:DOI:10042/to-9792
Cis-isomer LANL2DZ Advanced Optimization:DOI:10042/to-9794
Cis-isomer Frequency Analysis:DOI:10042/to-9795
Cis-isomer Advanced Frequency Analysis:DOI:10042/to-9796
Trans-isomer LANL2MB Optimization:DOI:10042/to-9797
Trans-isomer LANL2DZ Optimization:DOI:10042/to-9798
Trans-isomer LANL2DZ Advanced Optimization:DOI:10042/to-9799
Trans-isomer Frequency Analysis:DOI:10042/to-9800
Trans-isomer Advanced Frequency Analysis:DOI:10042/to-9801
In this task, cis- and trans- isomers of Mo(CO)4L2 was optimized and their IR spectra were calculated for analysis. First, the ground state structure of both cis and trans Mo(CO)4(PCl3)2 were created in GaussView 5.0, and roughly optimized using B3LYP/LANL2MB with "opt=loose" in the "Addtional key" box. The some bonds disappeared after optimization, but they did exist though they were longer than the typical bond lengths. This optimizations were slightly adjusted as the following:
Then the structures underwent a second optimization using B3LYP/LANL2DZ, with "int=ultrafine scf=cover=9" in the "Additional key" box, in order to obtain a more accurate result. At the same time, advanced LANL2DZ optimization, in which DAOs were taken into considerations, were run. Both results were displayed in the table for comparison.
Next, the frequency of these isomers were calculated using the same method, with "int=ultrafine scf=cover=9" in the "Additional key" box. The total energies unchanged after this vibration computation, and the optimizations were proved to be at the minimun energy points as expected.
| Optimization | Bond lengths | Bond Angles | Total Energy | Point Group | Others | |
|---|---|---|---|---|---|---|
| normal | P-Cl=2.24Å, P-Mo=2.51Å, C(equatorial)-Mo=2.01Å, C(axial)-Mo=2.05Å, C-O=1.17Å | Cl-P-Cl=98.9o, P-Mo-C(equatorial neighbour))=89.4o, Cl-P-Mo=118.2o, P-Mo-P=94.2o, P-Mo-C(axial)=89.2o | -623.57707194±0.00381 a.u. | C1 | The P-Cl and P-Mo bonds disappeared as the bond lengths were longer than that on the internal list of GaussView | |
| advanced | P-Cl=2.24Å, P-Mo=2.51Å, C(equatorial)-Mo=2.01Å, C(axial)-Mo=2.06Å, C-O=1.17Å | Cl-P-Cl=99.4o, P-Mo-C(equatorial neighbour))=89.4o, Cl-P-Mo=118.2o, P-Mo-P=94.2o, P-Mo-C(axial)=91.9o | -623.57707194±0.00381 a.u. | C1 | same as above | |
| normal | P-Cl=2.24Å, P-Mo=2.44Å, C-Mo=2.05Å, C-O=1.17Å | Cl-P-Cl=99.5o, P-Mo-C=90.0o, Cl-P-Mo=117.3o, P-Mo-P=117.3o | -623.57603111±0.00381 a.u. | C1 | The P-Cl bonds disappeared as the bond lengths were longer than that on the internal list of GaussView | |
| advanced | P-Cl=2.24Å, P-Mo=2.44Å, C-Mo=2.06Å, C-O=1.17Å | Cl-P-Cl=99.5o, P-Mo-C=91.3o, Cl-P-Mo=120.3o, P-Mo-P=117.4o | -623.57603111±0.00381 a.u. | C1 | same as above | |
According to the CHARACTERISTIC BOND LENGTHS IN FREE MOLECULES, CRC HANDBOOK OF CHEMISTRY, P-Cl=2.04Å which was smaller than the results of both isomers, C=O=1.21Å which was larger than the results of both isomers, and Mo-C=2.06Å, which was larger than that of cis-isomer but the same as that of trans-isomer. Generally, these values were close to that of the results, and therefore the optimizations were considered to be reasonable.
For the same isomer, he results of the normal and advanced LANL2DZ optimization were similar. The total energies unchanged, though there were some changes in bond lengths and bond angles.
For cis-tran isomers, they had very close bond lengths, bond angles and energy, but the trans-isomer seemed to have smaller structure as the most bulky groups PCl3 were closer to Mo than those in the cis-isomer. The cis-isomer had lower energy though the trans- isomer was considered to be thermodynamically more stable due to steric effect. The energy difference was 0.00104083 a.u., which was about 2.73269937 kJ/mol. This energy difference was small, and might be due to error.
In a molecule, atoms are in their vibrational ground state at absolute zero. But when the temperature is high enough, the atoms can be excited thermally and vibrate faster. The population of thermally excited atoms can be calculated using exp(-ΔE/kT) , where ΔE is the vibrational excitation energy, k is the Boltzmann constant and T is the temperature. Therefore, vibrations with very low frequency (that is, very low energy) can be excited easily at room temperature.
IR spectra of both isomers were computed by Gaussview. The one of the cis- isomer was more complicated, especially C=O stretching. That was because the structure of trans- isomer is much more symmetric, and all the CO groups have identical environment, so they absorb the same energy for vibration and give a singlet in the IR spectrum. For the cis-isomer, every CO group has different bond lengths as well as bond angles, and suffers different intramolecular forces, so they give peaks at four different positions in the IR spectrum. These C=O stretchings were compared with the experimental results in my 2nd Year Synthesis Lab (Spring) 4S lab reoprt. These results roughly matched though the experimental cis- isomer IR spectrum has only three C=O stretches appeared (the fourth might be overlapped by the others) and the frequencies were generally lower than the computational ones.
| Isomer | C=O Stretch / cm-1 | Experimental C=O Strethc / cm-1 |
|---|---|---|
| Cis | 1945.32, 1948.71, 1958.38, 2023.35 | 1884.06(double peaks), 2013.77 |
| Trans | 1950.28 | 1890.17 |
| IR Spectrum of Cis-Mo(CO)4(PCl3)2 |
|---|
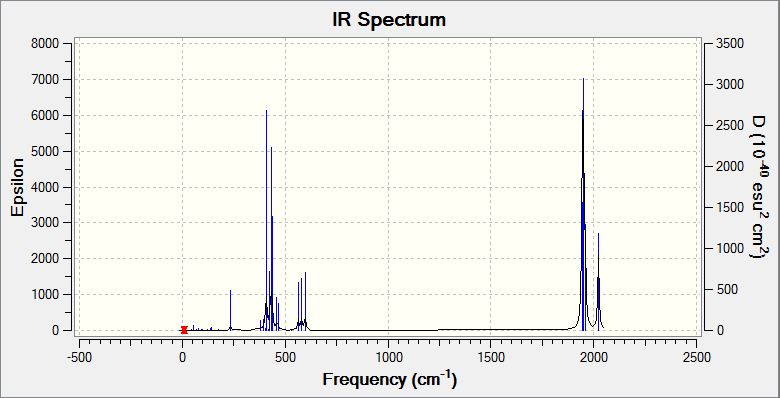
|
| IR Spectrum of Trans-Mo(CO)4(PCl3)2 |

|
References
1. Steric contributions to the solid-state structures of bis(phosphine) derivatives of molybdenum carbonyl. X-ray structural studies of cis-Mo(CO)4[PPh3-nMen2 (n = 0, 1, 2)], F. Albert Cotton, Donald J. Darensbourg, Simonetta Klein, and Brian W. S. Kolthammer, 1982,p294-299
2. Intramolecular hydrogen bonding and cation &pi-interactions affecting cis to trans isomerization in tungsten hexacarbonyl derivatives of 2-pyridyldiphenylphosphane and triphenylphosphane, Leeni Hirsivaara, Matti Haukka and Jouni Pursiainen, 2000, p508-510
3. The crystal and molecular structure of trans-tetracarbonylbis(triphenyl-phosphine)chromium(0) in a new unit cell: Is the trans conformer more stable than the cis?, Dennis W. Bennett, Tasneem A. Siddiquee, Daniel T. Haworth, Shariff E. Kabir and Farzana Camellia. J. Chem. Cryst., 2004, p353-359
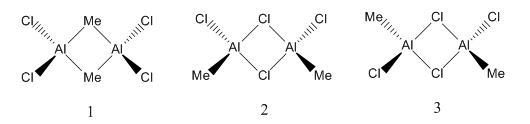
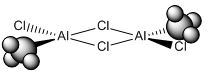
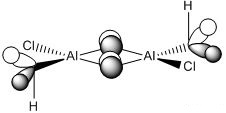
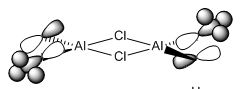
* Mini Project: MeAlCl2 Dimer
1st Optimization of 1:DOI:10042/to-9897
1st Optimization of 2:DOI:10042/to-9902
1st Optimization of 3:DOI:10042/to-9903
2nd Optimization of 1:DOI:10042/to-9812
2nd Optimization of 2:DOI:10042/to-9815
2nd Optimization of 3:DOI:10042/to-9817
Frequency Analysis of 1:DOI:10042/to-9818
Frequency Analysis of 2:DOI:10042/to-9820
Frequency Analysis of 3:DOI:10042/to-9821
MO Energy Calculation of 1:DOI:10042/to-9964
MO Energy Calculation of 2:DOI:10042/to-9965
MO Energy Calculation of 3:DOI:10042/to-9966
As mentioned in Dr. Lickiss's course, Main Group Chemistry (year 2), R2AlX and RAlX2, where R represents alkyl groups and X represents halide atoms, usually exists as dimer in non-polar solvents. And the interactions with heteroatom lone pairs are always more favoured than Al-C-Al bridging. So the aim of this mini project was to prove this using computational methods and find out the most stable structure of MeAlCl2 dimer.
In GaussView 5.0, the following structures were created and roughly optimized using DFT/B3LYP/3-21G, and then optimised again using DFT/B3LYP/6-31G(d). The resulted optimizations were checked to be at minimum energy point using frequency calculation.
| Structure | Bond lengths | Bond Angles | Total Energy | Point Group | Total Energy of Frequency Analysis | Others | |
|---|---|---|---|---|---|---|---|
| optimization | Cl-Al=2.11Å, C-Al=2.12Å, C-H=1.10Å | Cl-Al-Cl=118.6o, C-Al-C=105.6o, Cl-Al-C=108.5o, Al-C-Al=74.3o, H-C-H=102.7o | -2405.79471517±0.00381 a.u. | C1 | -2405.79471517±0.00381 a.u. | The bond between the two Al atoms appeared after optimization, that was because the optimized Al-Al distance is smaller than the Al-Al bond length in Gaussview's internal list. | |
| optimization | Cl(side)-Al=2.11Å, C-Al=1.94Å, Al-Cl(center)=2.32Å, C-H=1.10Å | Cl(side)-Al-Cl(center)=107.2o, C-Al-Cl(side)=123.1o, C-Al-Cl(center)=111.2o, Cl(centre)-Al-Cl(center)=89.5o, Al-Cl(center)-Al=90.5o, H-C-H=107.9o | -2405.82344448±0.00381 a.u. | C1 | -2405.82344448±0.00381 a.u. | ||
| optimization | Cl(side)-Al=2.12Å, C-Al=1.94Å, Al-Cl(center)=2.32Å, C-H=1.09Å | Cl(side)-Al-Cl(center)=107.4o, C-Al-Cl(side)=127.5o, C-Al-Cl(center)=111.7o, Cl(centre)-Al-Cl(center)=89.7o, Al-Cl(center)-Al=90.3o, H-C-H=107.9o | -2405.82440840±0.00381 a.u. | C1 | -2405.82440840±0.00381 a.u. | ||
The energies obtained from the frequency analysis were the same as those of the optimization, and that meant these optimized structures were the right ones to be found. The value of bond lengths and bond angles and molecular structure were similar to those in literature(DOI:10.1524/zpch.1958.18.3_4.147 ), and therefore the results were considered to be reasonable. The structures with Cl atoms at bridging positions were more stable as they had lower total energies. The energy of structure 1 was 75.428809151 kJ/mol higher than that of structure 2, and 77.959581304 higher than that of structure 3. So obviously structure 3 was the most stable.
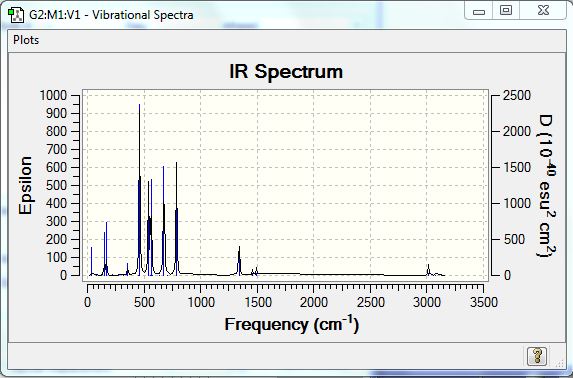
* Vibrational Spectra Analysis
The three vibrational spectra were similar, but the stretch positions slightly varied as bonding and geometries os structures were different. The table below showed IR spectroscopic details of the most stable structure 3, and the comparison between the three spectra.
* Molecular Orbital Analysis
In GaussView, the *.fch from of molecular orbital energy calculation result of structure 3 was opened. Since the total number of valence electron of the whole molecule was 48, 24 occupied MOs and LUMO, LUMO+1, LUMO+2 were calculated.
Five of these Mos were compared with their LCAO forms
* NBO Analysis
Above was the charge distribution results. In the .log file of the energy computation, more about the electronic structure could be found.
These three structures had different bond compositions. This was caused by structural differences. The following was part from the .log files:
| structure 1 | ||
|---|---|---|
| . Al-Cl bond | ( 17.21%) 0.4149*Al 1 s( 30.27%)p 2.23( 67.55%)d 0.07( 2.19%) | ( 82.79%) 0.9099*Cl 3 s( 32.30%)p 2.09( 67.47%)d 0.01( 0.24%) |
| Al-C bond | ( 11.76%)0.3430*Al 1 s( 19.97%)p 3.86( 77.13%)d 0.15( 2.90%) | ( 88.24%)0.9393* C 7 s( 31.33%)p 2.19( 68.66%)d 0.00( 0.02%) |
| Structure 2 | ||
| Al-Cl bond | ( 16.03%) 0.4004*Al 1 s( 26.28%)p 2.70( 71.07%)d 0.10( 2.65%) | ( 83.97%) 0.9163*Cl 3 s( 29.66%)p 2.36( 70.10%)d 0.01( 0.24%) |
| Al-Cl bond | ( 10.90%) 0.3301*Al 1 s( 17.66%)p 4.48( 79.17%)d 0.18( 3.17%) | ( 89.10%) 0.9439*Cl 5 s( 23.00%)p 3.34( 76.85%)d 0.01( 0.15%) |
| Al-C bond | ( 20.22%) 0.4496*Al 1 s( 38.92%)p 1.54( 59.83%)d 0.03( 1.25%) | ( 79.78%) 0.8932* C 8 s( 25.56%)p 2.91( 74.43%)d 0.00( 0.02%) |
| Structure 3 | ||
| Al-Cl bond | ( 10.89%) 0.3300*Al 1 s( 17.65%)p 4.48( 79.16%)d 0.18( 3.18%) | ( 89.11%) 0.9440*Cl 3 s( 22.95%)p 3.35( 76.90%)d 0.01( 0.15%) |
| Al-Cl bond | ( 15.87%) 0.3984*Al 1 s( 25.74%)p 2.78( 71.54%)d 0.11( 2.71%) | ( 84.13%) 0.9172*Cl 5 s( 29.72%)p 2.36( 70.04%)d 0.01( 0.24%) |
| Al-C bond | ( 20.41%) 0.4518*Al 1 s( 39.43%)p 1.51( 59.36%)d 0.03( 1.20%) | ( 79.59%) 0.8921* C 7 s( 25.38%)p 2.94( 74.61%)d 0.00( 0.02%) |
The "Second Order Perturbation Theory Analysis of Fock Matrix in NBO Basis" part did not have much to show for molecules in this task, as non of the energies exceeded 20kJ/mol.
In the "Natural Bond Orbital (Summary)" part, the energy and population or occupation of bonds and lone pairs were displayed. These results still different for the three structures.
* References
1. I.R. Spectroscopic Study of the components of the Ziegler Catalyst System TiCl4 + Al(CH3)2Cl, M.P.Groenewege, 1958