Rep:Mod:YYL0508miniproject
Loh Yong Yao
CID: 00605583
Lewis Acids and Bases Mini Project
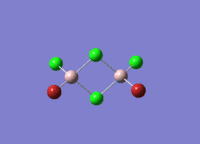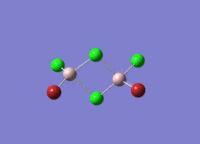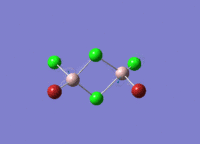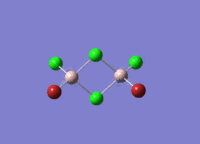
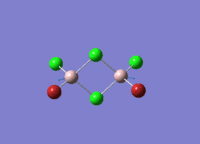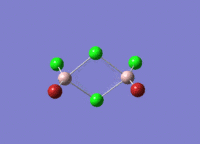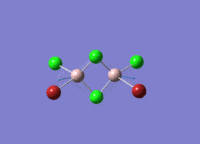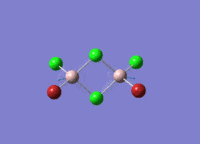
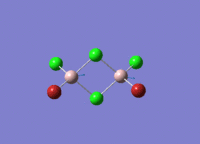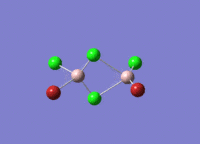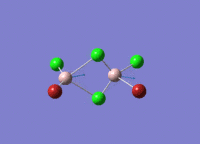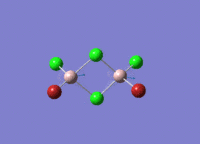
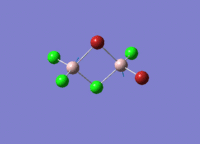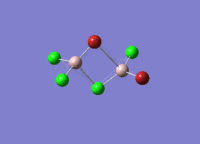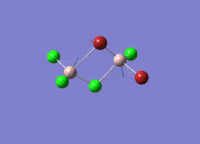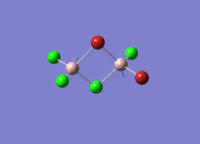
This mini project is about main group halides, specifically Al2Cl4Br2.
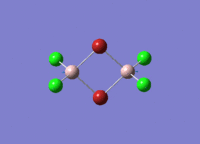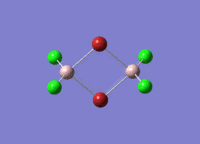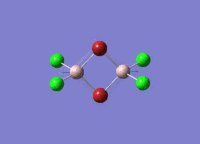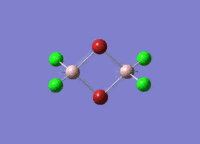
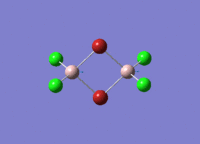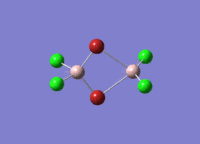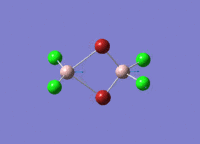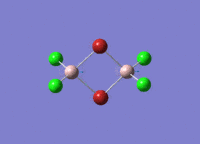
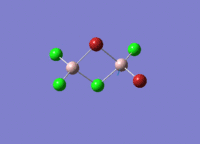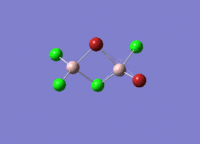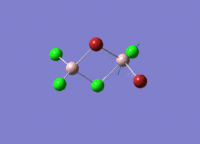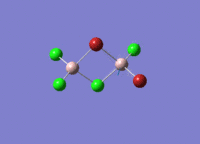
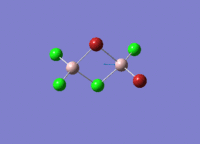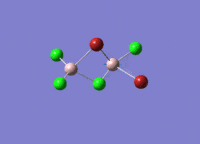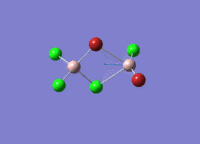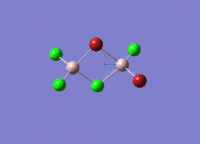
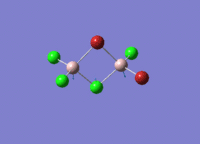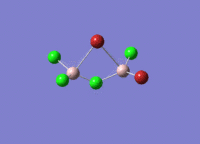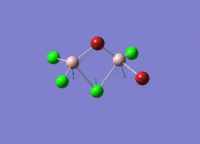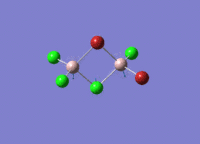
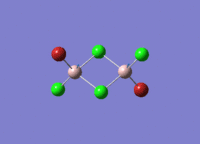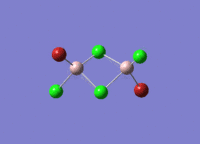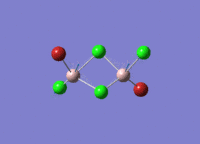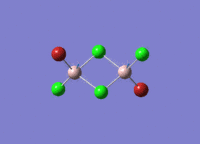
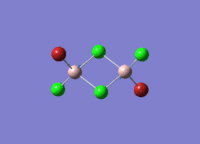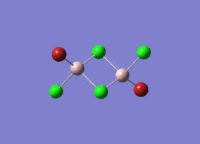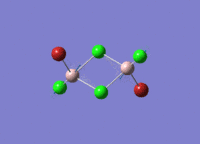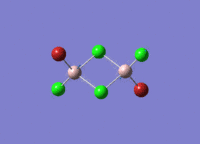
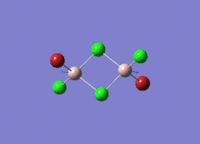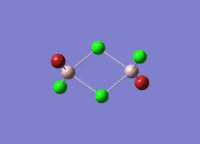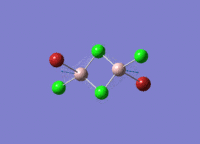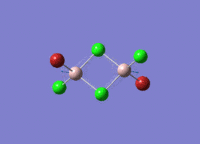
Isomers of Al2Cl4Br2
There are four isomers of Al2Cl4Br2 starting from AlCl2Br monomers and they are shown in the diagram below:
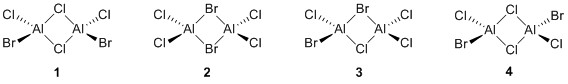
The symmetry of the isomers are summarized in the following table:
Optimisation of the 4 Isomers
The energies of all 4 isomers were optimised using a full basis set 6-31G(d,p) on Al and Cl and a PP LANL2DZdp on Br.
Isomer 1
Link to Log File for GEN Optimisation
Link to Dspace
The summary of the optimisation is as follows:
File type: .log
Calculation Type: FOPT
Calculation Method: RB3LYP
Basis Set: Gen
Final Energy: -2352.41626277 a.u.
Gradient: 0.00001536 a.u.
Dipole Moment: 0.18 D
Point Group: C2v
Job Time: 3 min 7.4 s
The optimisation is confirmed to have converged:
Item Value Threshold Converged?
Maximum Force 0.000017 0.000450 YES
RMS Force 0.000010 0.000300 YES
Maximum Displacement 0.000995 0.001800 YES
RMS Displacement 0.000295 0.001200 YES
Predicted change in Energy=-1.250811D-09
Optimization completed.
-- Stationary point found.
Isomer 2
Link to Log File for GEN Optimisation
Link to Dspace
The summary of the optimisation is as follows:
File type: .log
Calculation Type: FOPT
Calculation Method: RB3LYP
Basis Set: Gen
Final Energy: -2352.40631696 a.u.
Gradient: 0.00000879 a.u.
Dipole Moment: 0.00 D
Point Group: D2h
Job Time: 3 min 15.4 s
The optimisation is confirmed to have converged:
Item Value Threshold Converged?
Maximum Force 0.000029 0.000450 YES
RMS Force 0.000011 0.000300 YES
Maximum Displacement 0.000679 0.001800 YES
RMS Displacement 0.000282 0.001200 YES
Predicted change in Energy=-1.430828D-08
Optimization completed.
-- Stationary point found.
Isomer 3
Link to Log File for GEN Optimisation
Link to Dspace
The summary of the optimisation is as follows:
File type: .log
Calculation Type: FOPT
Calculation Method: RB3LYP
Basis Set: Gen
Final Energy: -2352.41110443 a.u.
Gradient: 0.00001133 a.u.
Dipole Moment: 0.14 D
Point Group: C1
Job Time: 3 min 32.8 s
The optimisation is confirmed to have converged:
Item Value Threshold Converged?
Maximum Force 0.000017 0.000450 YES
RMS Force 0.000009 0.000300 YES
Maximum Displacement 0.000365 0.001800 YES
RMS Displacement 0.000123 0.001200 YES
Predicted change in Energy=-9.106932D-09
Optimization completed.
-- Stationary point found.
Isomer 4
Link to Log File for GEN Optimisation
Link to Dspace
The summary of the optimisation is as follows:
File type: .log
Calculation Type: FOPT
Calculation Method: RB3LYP
Basis Set: Gen
Final Energy: -2352.41630427 a.u.
Gradient: 0.00003191 a.u.
Dipole Moment: 0.00 D
Point Group: C2h
Job Time: 2 min 38.0 s
The optimisation is confirmed to have converged:
Item Value Threshold Converged?
Maximum Force 0.000050 0.000450 YES
RMS Force 0.000018 0.000300 YES
Maximum Displacement 0.000361 0.001800 YES
RMS Displacement 0.000170 0.001200 YES
Predicted change in Energy=-1.578592D-08
Optimization completed.
-- Stationary point found.
Summary
The isomer with the lowest energy is Isomer 4, with an energy of - 6176269.007 kJ mol-1
| Isomer | Energy (kJ mol-1) | Energy relative to Isomer 4 (kJ mol-1) |
|---|---|---|
 |
||
 |
||
 |
||
 |
When Br is in a bridging position, the isomer is less stable. This is shown by the order of stability of the isomers in the table above. The most unstable isomer is the one where there are two bridging Brs, and the second most unstable isomer is the one which has one bridging Br and one bridging Cl. This is because Al-Br bonds are weaker than Al-Cl bonds due to a less effective overlap between Al and Br, which are in different rows on the periodic table, as compared to Al and Cl, which are in the same row. Hence, placing Br in terminal positions would minimize the number of weak Al-Br bonds and lower the energy of the compound.
Comparing the two most stable isomers which have two bridging Cls, the compound is most stable when the Brs are trans to each other. This is probably due to steric effects, as the Br atom is large as compared to the Cl atom and hence, placing Br atoms cis to each other would result in steric hindrance, destabilising the molecule. Hence, the most stable isomer is the one where Br atoms are terminal and trans to each other.
Dissociation Energy of Isomer 4 into 2 AlCl2Br
Optimisation of AlCl2Br
The energies of AlCl2Br was optimised using a full basis set 6-31G(d,p) on Al and Cl and a PP LANL2DZdp on Br.
Link to Log File for GEN Optimisation
Link to Dspace
The summary of the optimisation is as follows:
File type: .log
Calculation Type: FOPT
Calculation Method: RB3LYP
Basis Set: Gen
Final Energy: -1176.19013696 a.u.
Gradient: 0.00001820 a.u.
Dipole Moment: 0.11 D
Point Group:
Job Time: 48.1 s
The optimisation is confirmed to have converged:
Item Value Threshold Converged?
Maximum Force 0.000039 0.000450 YES
RMS Force 0.000025 0.000300 YES
Maximum Displacement 0.000285 0.001800 YES
RMS Displacement 0.000166 0.001200 YES
Predicted change in Energy=-1.045136D-08
Optimization completed.
-- Stationary point found.
Calculation of Dissociation Energy
The dissociation energy can be calculated as follows:
E(Al2Cl4Br2) = -2352.41630427 a.u.
E(AlCl2Br) = -1176.19013696 a.u.
ΔE = E(Al2Cl4Br2) - 2 [E(AlCl2Br)] = -2352.41630427 a.u. - 2 (-1176.19013696 a.u.) = -0.03603035 a.u. = -94.59768393 kJ mol-1
The value calculated is the value for association energy. Hence, dissociation energy is +94.6 kJ mol-1
A positive value for dissociation energy indicates that the product is more stable than the isolated monomers. However,compared to typical dissociation energies, which have a value of +100 to +500 kJ mol-1, the dissociation energy for the product into its monomers is relatively low. Hence, the bonds holding the product together are relatively weak.
Frequency Analysis of the 4 Isomers
The frequencies of all 4 isomers were calculated using a full basis set 6-31G(d,p) on Al and Cl and a PP LANL2DZdp on Br.
Isomer 1
Link to Log File for GEN Optimisation
Link to Dspace
The summary of the frequency calculation is as follows:
File type: .log
Calculation Type: FREQ
Calculation Method: RB3LYP
Basis Set: Gen
Final Energy: -2352.41626277 a.u. (Exactly the same as in the optimisation)
Gradient: 0.00001537 a.u.
Dipole Moment: 0.18 D
Point Group:
Job Time: 3 min 13.4 s
The low frequencies are within an acceptable range:
Low frequencies --- -4.6150 -3.0771 -1.1614 -0.0035 -0.0031 -0.0029 Low frequencies --- 17.6475 50.9652 78.5820
Isomer 2
Link to Log File for GEN Optimisation
Link to Dspace
The summary of the frequency calculation is as follows:
File type: .log
Calculation Type: FREQ
Calculation Method: RB3LYP
Basis Set: Gen
Final Energy: -2352.40631696 a.u. (Exactly the same as in the optimisation)
Gradient: 0.00000881 a.u.
Dipole Moment: 0.00 D
Point Group:
Job Time: 3 min 20.0 s
The low frequencies are within an acceptable range:
Low frequencies --- -3.6182 -2.2069 -0.0035 -0.0030 -0.0029 2.1345 Low frequencies --- 15.1911 63.5174 86.0413
Isomer 3
Link to Log File for GEN Optimisation
Link to Dspace
The summary of the frequency calculation is as follows:
File type: .log
Calculation Type: FREQ
Calculation Method: RB3LYP
Basis Set: Gen
Final Energy: -2352.41110443 a.u. (Exactly the same as in the optimisation)
Gradient: 0.00001130 a.u.
Dipole Moment: 0.14 D
Point Group:
Job Time: 3 min 51.2 s
The low frequencies are within an acceptable range:
Low frequencies --- -3.0010 -1.1466 -0.0012 -0.0010 0.0009 2.6917 Low frequencies --- 16.4882 55.9597 79.9700
Isomer 4
Link to Log File for GEN Optimisation
Link to Dspace
The summary of the frequency calculation is as follows:
File type: .log
Calculation Type: FREQ
Calculation Method: RB3LYP
Basis Set: Gen
Final Energy: -2352.41630427 a.u. (Exactly the same as in the optimisation)
Gradient: 0.00003189 a.u.
Dipole Moment: 0.00 D
Point Group:
Job Time: 3 min 57.7 s
The low frequencies are within an acceptable range:
Low frequencies --- -5.8725 -1.5356 -0.0021 -0.0019 -0.0012 3.0429 Low frequencies --- 18.4420 49.0267 72.9347
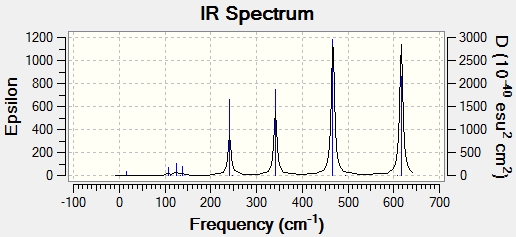
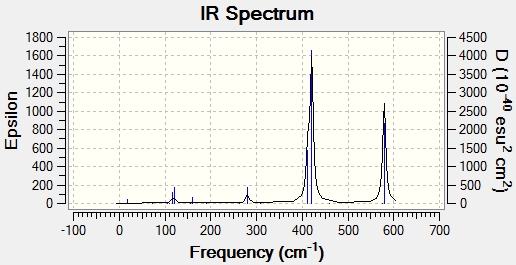
IR Spectra
The IR Spectra for the 4 different isomers are presented below:
IR Spectrum for Isomer 1
IR Spectrum for Isomer 2
IR Spectrum for Isomer 3
IR Spectrum for Isomer 4
Summary
The number of bands in the IR spectrum is related to the vibrational modes of the molecule. To show up as a band, the vibrational mode has to first involve a change in the dipole moment of the molecule. To show up as a distinct band, the vibrational mode must not be degenerate with any other vibrational mode. Hence, the number of bands in an IR spectrum is determined by the number of vibrational modes which involve a change in dipole moment of the molecule, and the number of non-degenerate vibrational modes. Hence, less symmetrical molecules are likely to have more bands in the IR spectrum as less symmetrical molecules are more likely to experience a change in dipole moment when stretching or bending, and are also less likely to have degenerate modes due to the asymmetry in the molecule.
Comparison of Al-Br Stretches Across the 4 Isomers
Isomer 1

Both Br are terminal in this isomer.
Isomer 2

Both Br are bridging in this isomer.
Isomer 3

1 Br is bridging amd 1 Br is terminal in this isomer.
Isomer 4

Both Br are terminal in this isomer.
Summary and Comparison
| Isomer | Position of Br | Frequency of vibration (cm-1) |
|---|---|---|
 |
||
 |
||
 |
1 Bridging |
|
 |
As can be seen from the tables above, the stretching frequencies for terminal Al-Br bonds are much higher compared to the stretching frequencies for bridging Al-Br bonds. Stretching frequency is a measure of bond strength. The higher the stretching frequency, the stronger the bond. The trend observed for the calculations can be rationalized when one considers the relative bond strengths. A terminal Al-Br bond is stronger as the electron density is spread across 2 atoms, as compared to a bridging Al-Br bond where the electron density is spread across 3 atoms. Hence, in Isomer 1 and 4, where there are only terminal Br atoms, one would expect to find only higher stretching frequencies between 400 to 600 cm-1. In Isomer 2, where there are only bridging Br atoms, one would expect to find only lower stretching frequencies between 170 to 370 cm-1. For Isomer 3, where there is one bridging Br and one terminal Br, a larger range of frequencies between 170 to 600 cm-1 is expected. These are consistent with the frequencies calculated in this exercise.
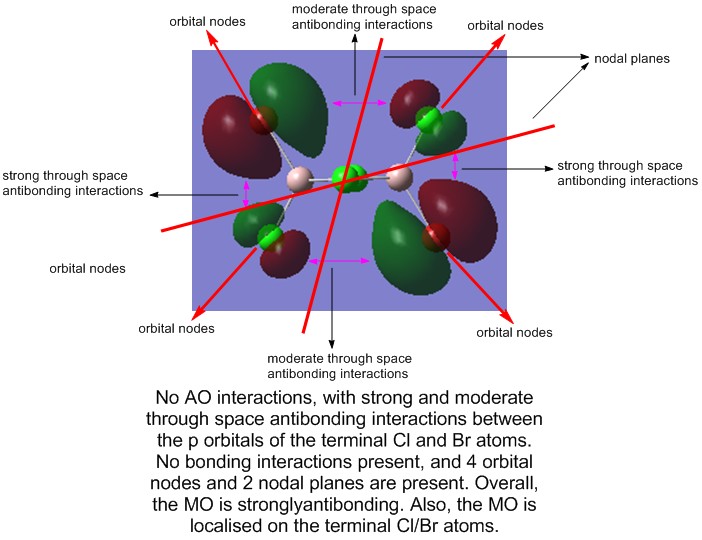
Molecular Orbitals of Isomer 4
The molecular orbitals of Isomer 4 were calculated using a full basis set 6-31G(d,p) on Al and Cl and a PP LANL2DZdp on Br.
Link for Log File for the calculation of MOs of Isomer 4
Dspace Link for calculation of MOs of Isomer 4
The non-core molecular orbitals are presented below:
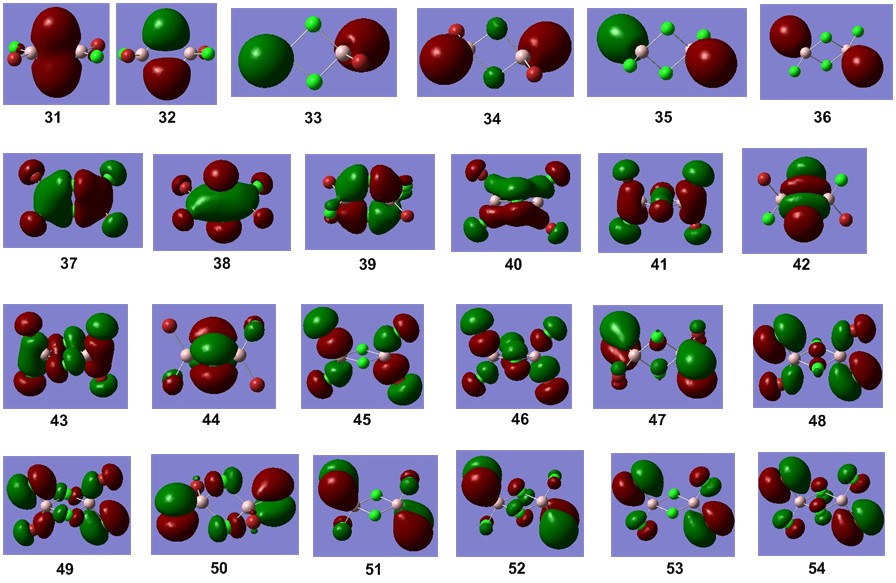
Presentation of 5 MOs
From the non-core MOs, 5 were selected for comparison and analysis. These are, in order of increasing antibonding nature, 31, 36, 37, 44, and 54.
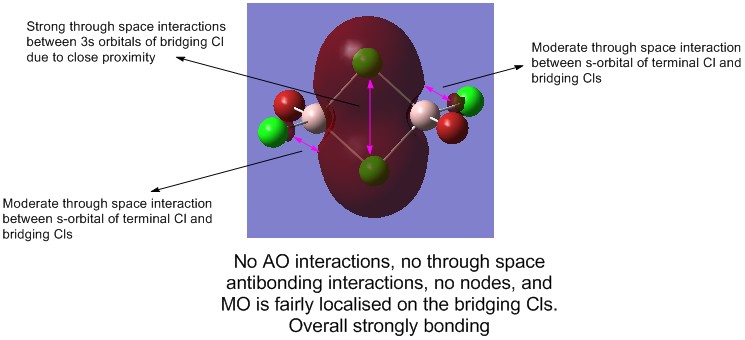
1) MO 31 (strongly bonding)
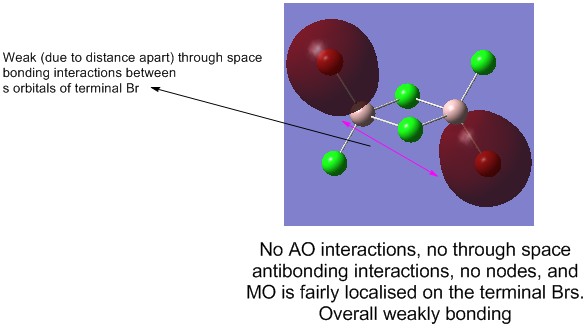
2) MO 36 (weakly bonding)
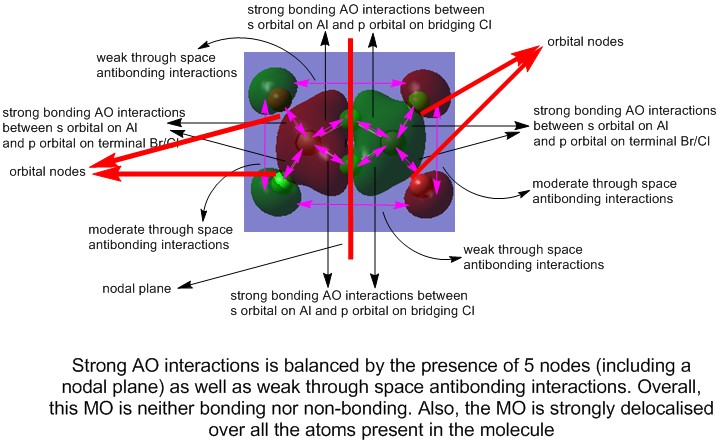
3) MO 37 (neither bonding nor antibonding)
4) MO 44 (weakly antibonding)
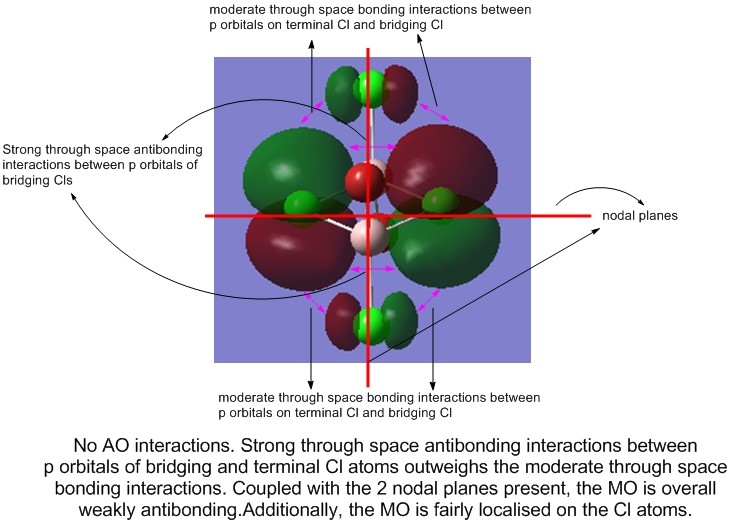
5) MO 53 (strongly antibonding)