Rep:Mod:YUDAOQIN
Advanced molecular modelling and assigning the absolute configuration of an epoxide
Conformational analysis using Molecular Mechanics
The Hydrogenation of Cyclopentadiene Dimer
Kinetic control VS Thermodynamic control
exo |
Dimer 1
exo |
Dimer 2
exo |
Dimer 3
exo |
Dimer 4
Cyclopentadiene dimerises to an endo dimer and an exo dimer, 1&2. Hydrogenation of these two dimers proceeds to give two relative derivatives 3&4. The aim is to distinguish which product undergoes a thermodynamic or a kinetic reaction. A series of chemical related tools are used. “Chembio3D” shows both planar and stereo versions of molecules. "Gaussview" uses to display predicted NMR spectra. Under the help of “Avogadro”, a few types of crucial energy obtain, such as total bond stretching energy, total angle bending energy, total stretch bending energy, total torsional energy, total out-of-plane bending energy, total Van der Waals energy, total electrostatic energy and total energy (sum) of a particular. These information are allocated and the table below lists them out.
| Property | Dimer 1 (kcal/mol) | Dimer 2 (kcal/mol) | Dimer 3 (kcal/mol) | Dimer 4 (kcal/mol) |
|---|---|---|---|---|
| Total bond stretching energy | 3.54360 | 3.46789 | 3.31086 | 2.93009 |
| Total angle bending energy | 30.77335 | 33.18937 | 31.92713 | 21.09197 |
| Total stretch bending energy | -2.04136 | -2.08218 | -2.10106 | -1.70918 |
| Total torsional energy | -2.73744 | -2.94947 | -1.46254 | 0.17128 |
| Total out-of-plane bending energy | 0.01469 | 0.02183 | 0.01315 | 0.00051 |
| Total Van der Waals energy | 12.80707 | 12.35873 | 13.63924 | 10.68113 |
| Total electrostatic energy | 13.01379 | 14.18450 | 5.11948 | 5.14891 |
| Total energy | 55.37368 | 58.19067 | 50.44627 | 38.31470 |
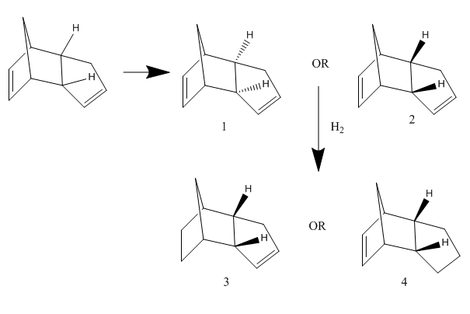
According to the table above, we clearly see that total energy (55.37368kcal/mol) of dimer 2 (55.37368kcal/mol) stays below while compare with dimer 1, which means dimer 1 is thermodynamic stable (ie: product stability) and dimer 2 is kinetic stable (ie: transition state stability). Likewise hydrogenated compound 3 gains more stability when it acts as a transition state and compound 4 prefers to act as a product. On the other hand, two indicated hydrogens senses more steric effect by the double bond in molecule 3, hence the higher total energy and less stable.

exo |
Intermediate9
exo |
Intermediate10
| Property | Intermediate 9 (kcal/mol) | Intermediate 10 (kcal/mol) |
|---|---|---|
| Total bond stretching energy | 7.70017 | 7.91408 |
| Total angle bending energy | 28.29773 | 20.97586 |
| Total stretch bending energy | -0.06950 | -0.06081 |
| Total torsional energy | 0.10769 | 4.45974 |
| Total out-of-plane bending energy | 0.98074 | 0.95594 |
| Total Van der Waals energy | 33.24245 | 34.75428 |
| Total electrostatic energy | 0.29497 | -0.04407 |
| Total energy | 70.55425 | 68.95502 |
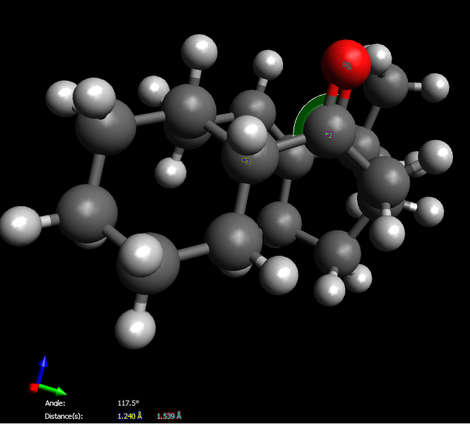
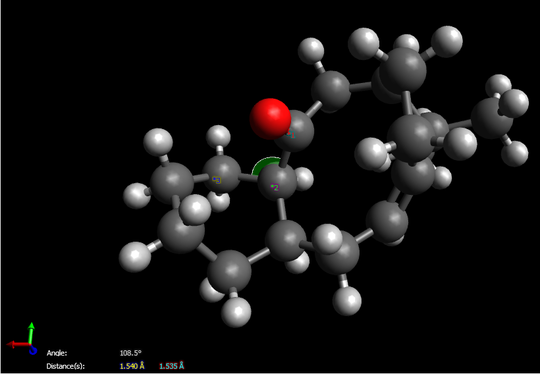
Due to total energy shown on this table, we easily determine molecule 10 is more stable since it owns relatively lower total energy. On the other hand, we find that all the energy between two intermediates are virtually the same expect for the total angle bending energy. By using “Avogadro”, we prove that the angle between C-O-C(carbon on six-membered ring) for compound 10 is 108.5。 which exactly matches the angle of sp3 hybridization, however, the angle distorts to 117.5。 for compound 9 while a large gap indicates. Therefore, the final total energy of molecule 9 remains at a relatively high level.
Here is the proof:
Futhermore, for the reason that why alkenes particular for 9 and 10 react slowly. A new class of “hyperstable” olefins can now be defined, olefins which contain less strain than that of the parent hydrocarbon and have negative OS values. Such olefins should be very unreactive-not due to steric hindrance3’ or to enhanced a-bond strength but due to special stability afforded by the cage structure of the olefin and to the greater strain of the parent polycycloalkane.[1]
Spectroscopic Simulation using Quantum Mechanics
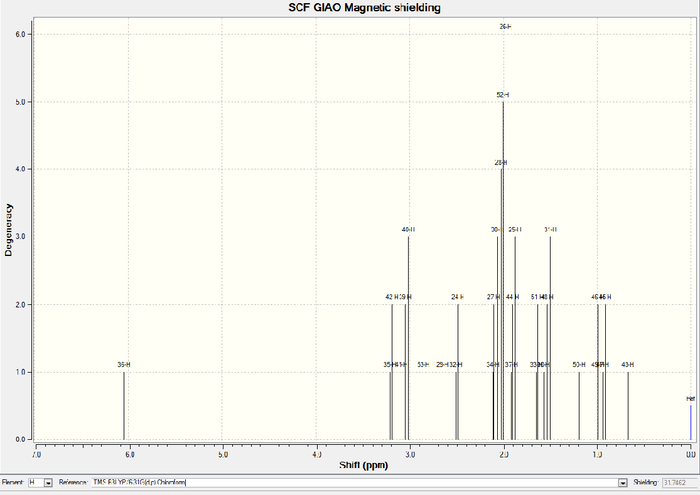

| chmeical shift (ppm) | Degeneracy | Atoms |
|---|---|---|
| 6.05 | 1 | 36 |
| 3.20 | 2 | 35,42 |
| 3.05 | 3 | 41,39,40 |
| 2.85 | 1 | 53 |
| 2.65 | 1 | 29 |
| 2.50 | 2 | 32,24 |
| 2.05 | 6 | 34,27,38,28,52,26 |
| 1.90 | 2 | 37,35 |
| 1.64 | 1 | 33 |
| 1.53 | 2 | 30,31 |
| 1.28 | 3 | 49 50 51 ave |
| 1.17 | 3 | 43 44 45 ave |
| 1.15 | 3 | 46 47 48 ave |
| chmeical shift (ppm) | Degeneracy | Coupling |
|---|---|---|
| 5.21 | 1 | multiplet |
| 3.00-2.70 | 6 | multiplet |
| 2.70-2.35 | 4 | multiplet |
| 2.20-1.70 | 6 | J=5.4HZ |
| 1.58 | 1 | multiplet |
| 1.50-1.20 | 3 | multiplet |
| 1.10 | 3 | singlet |
| 1.07 | 3 | singlet |
| 1.03 | 3 | singlet |
| chmeical shift (ppm) | Degeneracy | Atoms |
|---|---|---|
| 212.18 | 1 | 6 |
| 148.12 | 1 | 4 |
| 120.21 | 1 | 12 |
| 94.55 | 1 | 15 |
| 60.53 | 1 | 11 |
| 57.47 | 1 | 8 |
| 55.06 | 1 | 3 |
| 49.83 | 1 | 14 |
| 44.09 | 1 | 20 |
| 42.06 | 1 | 16 |
| 41.62 | 1 | 19 |
| 37.38 | 1 | 13 |
| 36.62 | 1 | 5 |
| 33.94 | 1 | 9 |
| 29.00 | 1 | 21 |
| 27.96 | 1 | 2 |
| 26.18 | 1 | 22 |
| 25.44 | 1 | 1 |
| 24.78 | 1 | 10 |
| 22.51 | 1 | 23 |
| chmeical shift (ppm) | Degeneracy | Atoms |
|---|---|---|
| 211.49 | 1 | 6 |
| 148.72 | 1 | 4 |
| 120.90 | 1 | 12 |
| 74.61 | 1 | 15 |
| 60.53 | 1 | 11 |
| 51.30 | 1 | 8 |
| 50.94 | 1 | 3 |
| 45.53 | 1 | 14 |
| 43.28 | 1 | 20 |
| 40.82 | 1 | 16 |
| 38.73 | 1 | 19 |
| 36.78 | 1 | 13 |
| 35.47 | 1 | 5 |
| 30.84 | 1 | 9 |
| 30.00 | 1 | 21 |
| 25.56 | 1 | 2 |
| 25.35 | 1 | 22 |
| 22.21 | 1 | 1 |
| 21.39 | 1 | 10 |
| 19.83 | 1 | 23 |
In 1H NMR of molecule 18, three easily distinguishable "ave" (average) list in table 1 since "Gaussview" always counts three Hs in methyl group as a singlet regardless of any other influences. Hence, find them out, average them and get the final result which is shown in table 1. In comparison with literature values of 1H NMR, main peaks almost match like degeneracy=6 and while chemical shift=1.58. Three methyl groups count a little distinction due to the systematic error for skip of adjacent electrogative atoms such as sulphur. There is about 1ppm deviation at carbonyl groups when compared with the referred values since two distinct solvents are used (CHCl3 and C6D6). Chloroform contains three very electronegative chlorine atoms to withdraw electron density and then leads to a more shielded carbonyl group.
Likewise, in table 3&4, only one exception appears. Carbon 15 in simulated spectrum directly connects with two sulphur atoms can be readily distorted since highly electronegative chlorine atoms in solvent contribute shielding effect in a huge extent. The site of sulphur attachment, carbon 15 is approximate 20 ppm too high (6-31G(d,p)/aug-cc-pVDZ bases, respectively). This is a well-known effect due to spin–orbit (SO) coupling and is exhibited increasingly by “heavy elements”. [3]

Thermal energy: The sum of electronic and thermal Energies= -1651.392841kJ/mol which stands by that the free energy is much less than zero. In another word, forward reaction will proceed spontaneous and the product is quite stable since a such a huge negative values gained.
Analysis of the properties of the synthesised alkene epoxides
The Shi asymmetric Fructose catalyst is usually used as a catalyst to speed up epoxidation of trans-alkenes. Species 21 is the stable precursor which can be isolated, and compound 22 normally acts as an active catalyst generated from 21 by persulfuric acid to catalyze trans-alkenes. Likewise, species 23 behaves as a pre-catalyst of Jacobsen asymmetric catalyst to maximize the speed and yield of a series of epoxidation of cis-alkenes.

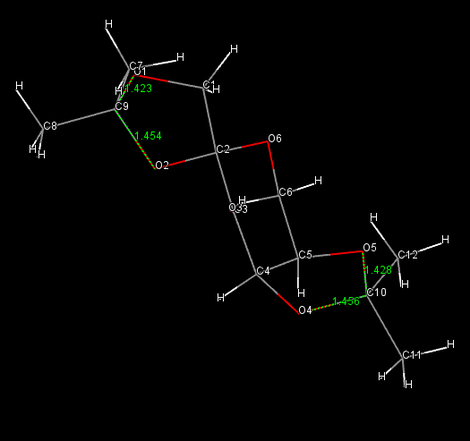
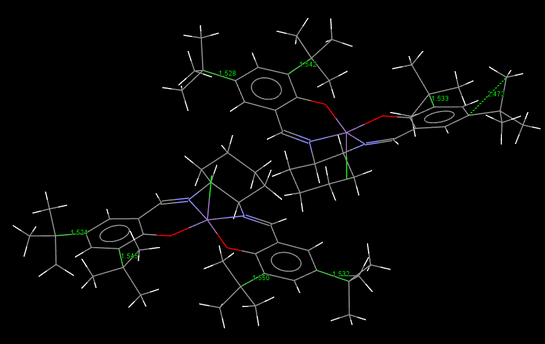
The crystal structures of the two catalysts
Left-handed picture shows the whole O-C-O angles for compound 21. Anomeric centres lead to the a difference between two C-O bond lengths since the antibonding orbital (π*) for the longer bond withdraws electron density from its neighbor, hence lengthen and weaken itself and stablise the adjacent one. An extreme high electronegative oxygen 2 next to carbon 9 cause the anomalous electron density withdrawing phenomenon. Although carbon 9 demonstrates sp3 hybridization, a hugely deviated anomeric angle (O-C-O), 90.1。 is discovered. Similarly, in molecule 23, it is clearly to see that oxygen-near bonds display lengthen tBu-C bonds. In contrast, adjacent tBu-C bonds own relatively shorter and stronger bonds.
The calculated NMR properties of styrene epoxide and trans-stilbene epoxide
| chmeical shift (ppm) | Degeneracy | Atom |
|---|---|---|
| 7.49 | 4 | 10,11,12,13 |
| 7.30 | 1 | 14 |
| 3.66 | 1 | 15 |
| 3.11 | 1 | 16 |
| 2.53 | 1 | 17 |
| chmeical shift (ppm) | Degeneracy | Atom |
|---|---|---|
| 135.13 | 1 | 5 |
| 124.13 | 1 | 1 |
| 123.41 | 1 | 3 |
| 122.96 | 2 | 4,2 |
| 118.27 | 1 | 6 |
| 54.06 | 1 | 7 |
| 53.47 | 1 | 8 |
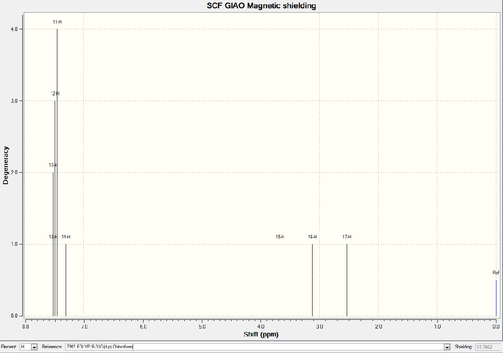
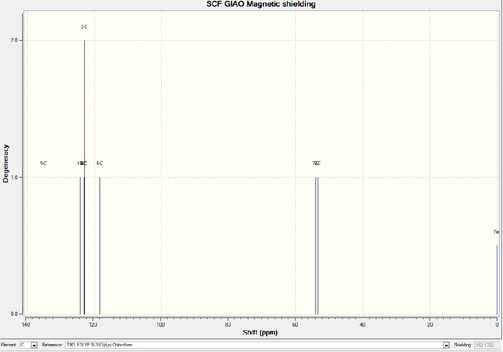
Five hydrogens stay on the aromatic ring display much higher chemical shift while compare with the aliphatic ones. Four of them, which occupy meta and ortho position, exert exact the same chemical environment. The para-hydrogen shows a lower chemical shift since it stays furthest way from the electronegative oxygen. The rest two non-aromatic hydrogens feels strong effect by oxygen, hence 3.11ppm as well as 2.53ppm interpret the shielding effect. For the 13C NMR, similar phenomenon occurs. Six aromatic carbons demonstrate much higher chemical shifts than the others. On the other hand, the diverse distances away from oxygen lead to the difference between these six carbons.
Coupling constant of styrene epoxide:
Total nuclear spin-spin coupling J (Hz):
1 2 3 4 5
1 0.000000D+00
2 0.571310D+02 0.000000D+00
3 -0.157408D+01 0.574268D+02 0.000000D+00
4 0.963412D+01 -0.196492D+01 0.586112D+02 0.000000D+00
5 -0.988936D+00 0.964645D+01 -0.131227D+01 0.599266D+02 0.000000D+00
6 0.590920D+02 -0.203077D+01 0.957116D+01 -0.771475D+00 0.601600D+02
7 0.349630D+01 -0.108482D+01 0.571813D+01 0.827746D+01 0.571068D+02
8 -0.777944D+00 0.690145D+00 -0.633260D+00 0.108609D+01 -0.103018D+01
9 -0.176398D+00 -0.850825D-03 -0.279767D+00 -0.254025D+00 -0.227208D+01
10 0.166984D+03 0.222699D+01 0.785275D+01 -0.139791D+01 0.762491D+01
11 0.230410D+01 0.167539D+03 0.231640D+01 0.795279D+01 -0.148990D+01
12 0.791557D+01 0.209187D+01 0.167131D+03 0.239406D+01 0.766331D+01
13 -0.115003D+01 0.756234D+01 0.160099D+01 0.164968D+03 0.118637D+01
14 0.194307D+01 0.791787D+01 -0.114864D+01 0.657710D+01 0.101130D-01
15 0.344508D+00 0.343638D+00 -0.396642D+00 0.308555D+01 0.601942D+01
16 -0.770151D-01 -0.430522D-02 -0.124604D+00 -0.407359D-01 0.952064D+00
17 0.133535D+00 -0.171306D+00 0.758510D-01 -0.156440D+00 0.201590D+01
6 7 8 9 10
6 0.000000D+00
7 0.957985D+00 0.000000D+00
8 0.133242D+01 0.273554D+02 0.000000D+00
9 -0.124815D+01 0.271457D+02 0.275527D+02 0.000000D+00
10 0.261141D+01 0.533225D+00 0.264815D+00 -0.318626D-01 0.000000D+00
11 0.782327D+01 0.666420D+00 -0.653879D+00 0.230496D-01 0.833435D+01
12 -0.138942D+01 0.811084D+00 0.221107D+00 -0.851157D-01 0.951482D+00
13 0.644446D+01 0.670708D+01 -0.654869D+00 0.143724D+00 0.427830D+00
14 0.167689D+03 0.449865D+01 -0.481929D+00 -0.331056D+00 0.891046D+01
15 0.380873D+01 0.184555D+03 -0.143714D+01 -0.584559D+01 0.442769D+00
16 -0.108933D+00 -0.131149D+01 0.183284D+03 -0.532709D+01 -0.121109D+00
17 -0.174809D+00 -0.233575D+01 0.183888D+03 -0.583976D+01 -0.835975D-01
11 12 13 14 15
11 0.000000D+00
12 0.840713D+01 0.000000D+00
13 0.837579D+00 0.868334D+01 0.000000D+00
14 0.916096D+00 0.445258D+00 0.153009D+01 0.000000D+00
15 -0.427111D+00 -0.342228D-01 -0.738427D-01 -0.791018D+00 0.000000D+00
16 -0.237492D+00 -0.116332D+00 -0.170454D+00 -0.250857D+00 0.442008D+01
17 -0.588924D-01 -0.175587D+00 -0.584888D-01 0.357692D+00 0.186391D+01
The data above list entire coupling constants of mutually elements.
| chmeical shift (ppm) | Degeneracy | Atom |
|---|---|---|
| 7.57 | 2 | 17,26 |
| 7.48 | 8 | 23,20,24,19,18,25,27,16 |
| 3.54 | 2 | 22,21 |
Similar to the 1H NMR of styrene epxodie, eight ortho- and meta- hydrogens exerts exact the same chemical environment. Two para-hydrogens shows the smaller chemical shift and the aliphatic hydrogens display 3.54ppm because of the connection with electrongative oxygen.
| chmeical shift (ppm) | Degeneracy | Atom |
|---|---|---|
| 134.09 | 2 | 9,16 |
| 124.22 | 2 | 2,13 |
| 123.52 | 2 | 11,4 |
| 123.21 | 2 | 3,12 |
| 123.07 | 2 | 5,10 |
| 118.26 | 2 | 11,4 |
| 66.42 | 2 | 8,7 |
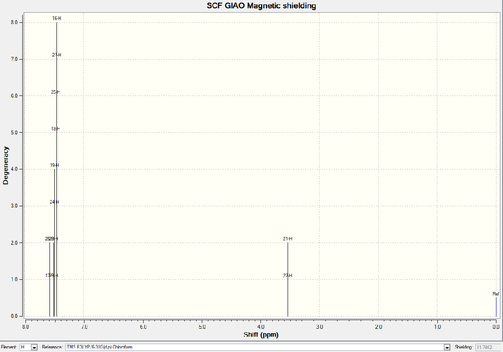
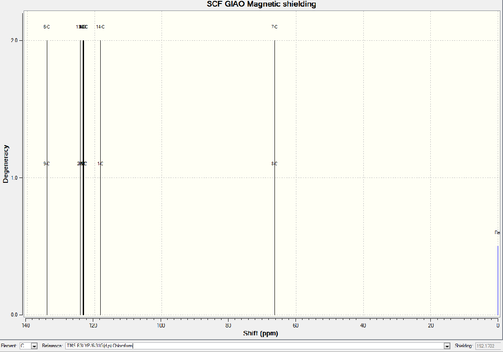
Paired aromatic carbons own much higher chemical shift than the non-aromatic ones. The distances between carbons and oxygen causes the difference among all 12 aromatic carbons.
Coupling constant of trans-stilbene epoxide:
Total nuclear spin-spin coupling J (Hz):
1 2 3 4 5
1 0.000000D+00
2 0.590241D+02 0.000000D+00
3 -0.202482D+01 0.571363D+02 0.000000D+00
4 0.954883D+01 -0.158098D+01 0.573741D+02 0.000000D+00
5 -0.799345D+00 0.963207D+01 -0.195421D+01 0.586312D+02 0.000000D+00
6 0.602104D+02 -0.971512D+00 0.966579D+01 -0.130918D+01 0.597618D+02
7 0.918426D+00 0.349050D+01 -0.110348D+01 0.568974D+01 0.829082D+01
8 0.126857D+01 -0.791116D+00 0.706118D+00 -0.653312D+00 0.104817D+01
9 0.307670D-01 -0.528051D-02 0.107791D-01 -0.374137D-01 -0.203564D-01
10 0.423394D-01 -0.115893D-01 0.583021D-02 -0.148643D-01 0.262618D-01
11 -0.374294D-02 0.653025D-02 -0.142616D-01 0.300898D-02 -0.146962D-01
12 -0.211705D-02 -0.159400D-01 -0.228317D-02 -0.135984D-01 0.514301D-02
13 0.597934D-03 0.321759D-02 -0.162134D-01 0.616203D-02 -0.112326D-01
14 0.130799D+00 0.872188D-03 -0.208582D-02 -0.313798D-02 0.419130D-01
15 -0.130676D+01 -0.178555D+00 -0.145763D-01 -0.257040D+00 -0.263369D+00
16 0.167893D+03 0.191927D+01 0.790433D+01 -0.114368D+01 0.655844D+01
17 0.262063D+01 0.167131D+03 0.222645D+01 0.784928D+01 -0.139275D+01
18 0.783207D+01 0.228964D+01 0.167613D+03 0.231912D+01 0.795531D+01
19 -0.138353D+01 0.790513D+01 0.210756D+01 0.167261D+03 0.238640D+01
20 0.643239D+01 -0.115020D+01 0.757860D+01 0.159797D+01 0.165336D+03
21 0.382152D+01 0.338049D+00 0.358043D+00 -0.402455D+00 0.302261D+01
22 -0.142823D+00 0.129179D+00 -0.166652D+00 0.854513D-01 -0.133010D+00
23 0.448375D-02 0.793561D-02 -0.172462D-01 -0.123744D-02 0.134119D-02
24 -0.441218D-02 -0.189057D-01 -0.114282D-01 -0.176667D-01 -0.450972D-02
25 -0.383387D-01 -0.759967D-02 -0.402309D-01 -0.504313D-02 -0.362720D-01
26 -0.163310D-01 -0.261653D-01 -0.184986D-01 -0.211967D-01 -0.992618D-02
27 -0.107181D-01 -0.988087D-02 -0.423388D-01 -0.102144D-01 -0.118536D-01
6 7 8 9 10
6 0.000000D+00
7 0.565004D+02 0.000000D+00
8 -0.921093D+00 0.269017D+02 0.000000D+00
9 -0.906751D-02 -0.920928D+00 0.565008D+02 0.000000D+00
10 -0.211558D-01 0.104774D+01 0.829082D+01 0.597616D+02 0.000000D+00
11 -0.367578D-01 -0.653144D+00 0.569000D+01 -0.130989D+01 0.586295D+02
12 0.104240D-01 0.705642D+00 -0.110373D+01 0.966607D+01 -0.195211D+01
13 -0.499089D-02 -0.790828D+00 0.349084D+01 -0.972181D+00 0.963109D+01
14 0.308135D-01 0.126836D+01 0.917867D+00 0.602111D+02 -0.798788D+00
15 -0.203320D+01 0.276403D+02 0.276402D+02 -0.203298D+01 -0.263368D+00
16 0.802313D-01 0.445976D+01 -0.462426D+00 -0.155404D-01 -0.113485D-01
17 0.764067D+01 0.524351D+00 0.287944D+00 -0.584136D-02 -0.992552D-02
18 -0.148313D+01 0.675827D+00 -0.660731D+00 -0.411105D-01 -0.368709D-01
19 0.768561D+01 0.828058D+00 0.232355D+00 -0.220431D-01 -0.498588D-02
20 0.122291D+01 0.661535D+01 -0.650071D+00 -0.160079D-01 0.641622D-03
21 0.621889D+01 0.185757D+03 -0.198712D+01 0.171614D+01 -0.132522D+00
22 0.171584D+01 -0.198712D+01 0.185757D+03 0.621870D+01 0.302249D+01
23 -0.155024D-01 -0.649858D+00 0.661543D+01 0.122230D+01 0.165335D+03
24 -0.218052D-01 0.232346D+00 0.827672D+00 0.768621D+01 0.238747D+01
25 -0.405467D-01 -0.660364D+00 0.676265D+00 -0.148377D+01 0.795375D+01
26 -0.581180D-02 0.287866D+00 0.524304D+00 0.764081D+01 -0.139250D+01
27 -0.155340D-01 -0.462288D+00 0.446045D+01 0.800687D-01 0.655777D+01
11 12 13 14 15
11 0.000000D+00
12 0.573734D+02 0.000000D+00
13 -0.158108D+01 0.571360D+02 0.000000D+00
14 0.954930D+01 -0.202480D+01 0.590247D+02 0.000000D+00
15 -0.256969D+00 -0.147857D-01 -0.178344D+00 -0.130710D+01 0.000000D+00
16 -0.999333D-02 -0.417066D-01 -0.984193D-02 -0.108380D-01 -0.331476D+00
17 -0.211970D-01 -0.186152D-01 -0.260982D-01 -0.163298D-01 -0.382960D-01
18 -0.472411D-02 -0.399812D-01 -0.753000D-02 -0.378004D-01 0.363926D-01
19 -0.177648D-01 -0.121180D-01 -0.188698D-01 -0.433133D-02 -0.898954D-01
20 -0.927718D-03 -0.171572D-01 0.774447D-02 0.499666D-02 0.163628D+00
21 0.852632D-01 -0.166315D+00 0.129249D+00 -0.142993D+00 -0.597485D+01
22 -0.402397D+00 0.357920D+00 0.337891D+00 0.382152D+01 -0.597482D+01
23 0.159806D+01 0.757815D+01 -0.115036D+01 0.643294D+01 0.163796D+00
24 0.167261D+03 0.210819D+01 0.790521D+01 -0.138377D+01 -0.901694D-01
25 0.231944D+01 0.167613D+03 0.228924D+01 0.783260D+01 0.365167D-01
26 0.784927D+01 0.222660D+01 0.167131D+03 0.262053D+01 -0.383603D-01
27 -0.114365D+01 0.790390D+01 0.191915D+01 0.167893D+03 -0.331233D+00
16 17 18 19 20
16 0.000000D+00
17 0.890181D+01 0.000000D+00
18 0.921591D+00 0.835168D+01 0.000000D+00
19 0.449771D+00 0.950874D+00 0.840337D+01 0.000000D+00
20 0.153903D+01 0.440186D+00 0.846249D+00 0.869871D+01 0.000000D+00
21 -0.768939D+00 0.460609D+00 -0.421301D+00 -0.155033D-01 -0.455100D-01
22 0.394449D+00 -0.583921D-01 -0.358106D-01 -0.155615D+00 -0.170080D-01
23 0.437218D-01 -0.267419D-01 -0.380829D-01 -0.438095D-01 0.958478D-02
24 -0.611162D-01 -0.545318D-01 -0.637346D-01 -0.485831D-01 -0.438086D-01
25 -0.699569D-01 -0.749112D-01 -0.578030D-01 -0.637168D-01 -0.380805D-01
26 -0.712479D-01 -0.691284D-01 -0.749064D-01 -0.545282D-01 -0.267281D-01
27 -0.956643D-02 -0.712561D-01 -0.699685D-01 -0.611165D-01 0.437039D-01
21 22 23 24 25
21 0.000000D+00
22 0.119747D+01 0.000000D+00
23 -0.170055D-01 -0.455569D-01 0.000000D+00
24 -0.155566D+00 -0.154490D-01 0.869873D+01 0.000000D+00
25 -0.358310D-01 -0.421367D+00 0.846221D+00 0.840337D+01 0.000000D+00
26 -0.583959D-01 0.460628D+00 0.440199D+00 0.950871D+00 0.835169D+01
27 0.394411D+00 -0.768999D+00 0.153899D+01 0.449784D+00 0.921553D+00
26 27
26 0.000000D+00
27 0.890182D+01 0.000000D+00
The data above list entire coupling constants of mutually elements.
Assigning the absolute configuration of the product
In order to determine the configuration of styrene epoxide, we need to determine which catalyst will be used. Since one side of the double bond in styrene are occupied by two same hydrogens, each of the catalyst can be chosen. Shi catalyst, which is used to do the epoxidation of trans- alkene, will catalyze styrene to produce styrene epoxide.
The Shi epoxidation is a chemical reaction described as an asymmetric epoxidation of olefins with oxone (potassium peroxymonosulfate) and a fructose-derived catalyst (1). It is notable as not requiring metal catalysts. The mechanism involves the formation of a dioxirane intermediate.[4][5]
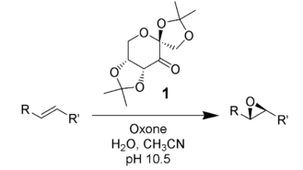
This procedure generates epoxides with high enantiomeric excesses from trans-disubstituted alkenes and trisubstituted alkenes. Styrenes[6] are asymmetrically epoxidized using the same catalyst.
So, the optical rotation of styrene epoxide is like the picture——configuration of styrene epoxide:

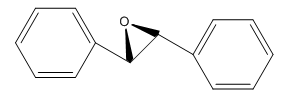
For the trans-stilbene, it proceeds exact the same way to produce trans-stilbene epoxide. Just like the image below:
Investigating the non-covalent interactions in the active-site of the reaction transition state
I choose the forth transition state in R,R series.[8]
Non-covalent interactions (NCI) include hydrogen bonds, electrostatic attractions and dispersion-like close approaches of pairs of atoms. As for normal covalent bonds, such NCI interactions can be defined by the properties of the electron density (and its curvatures). The colors indicate whether the interaction is attractive or not (colours: blue = very attractive, green = mildly attractive, yellow = mildly repulsive, red = strongly repulsive).[7]
It is clear to see that green color, that means dispersion force dominates, following by the yellow one. The area covers by blue (very attractive) and red (very replusive) seem to less than 5%. In another word, this molecule is quite stable as a result of the minimization of intermolecular forces except for the anomeric effect. O-C-O bonds are distorted away from the normal structure like sp3 hybridization orbital, no other aspect distorts the molecule.
Orbital |
Investigating the Electronic topology (QTAIM) in the active-site of the reaction transition state
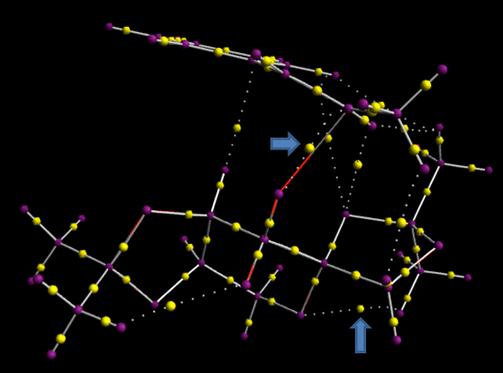
The bottom arrow indicates a non-covalent BCP which is the interaction between oxygen and hydrogen due to the huge electronegativity difference. The horizontal arrow at middle displays a bond between oxygen and carbon. Clearly, the dotted line forms a curvature and the BCP stay on it. Similarly, a few of dispersion forces, connect the upper and bottom layer, will contort original shape visibly.
Optical rotation power
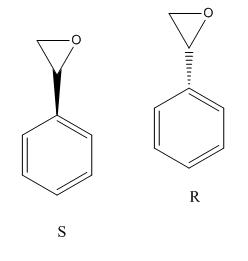
Name: (R)-2-phenyloxirane Optical rotatory power: -24 deg [10]
Name: (S)-(-)-styrene oxide Optical rotatory power: 4.3 deg [11]

Name: cis R-(+)-pulegone oxide Optical rotatory power: 853.9 deg [12]
Name: pulegone epoxide Optical rotatory power: 786.5 deg [12]
References
[1]: W. F. Maier, P. Von Rague Schleyer, J. Am. Chem. Soc., 1981, 103, 1891.
[2]: Spectroscopic data: L. Paquette, N. A. Pegg, D. Toops, G. D. Maynard, R. D. Rogers, J. Am. Chem. Soc.,, 1990, 112, 277-283
[3]: Kaupp, M.; Malkina, O.; Malkin, V. G. Chem. Phys. Lett. 1997, 265, 55–59.
[4]: An Efficient Catalytic Asymmetric Epoxidation Method Zhi-Xian Wang, Yong Tu, Michael Frohn, Jian-Rong Zhang, and Yian Shi J. Am. Chem. Soc. 1997, 119(46), 11224-11235.
[5]: Frohn, M.; Shi, Y. Synthesis 2000, 14, 1979-2000 doi:10.1055/s-2000-8715.
[6]: Tian, H.; She, X.; Xu, J.; Shi, Y. Org. Lett. 2001, 3, 1929-1931.
[7]: J. L. Arbour, H. S. Rzepa, J. Contreras-García, L. A. Adrio, E. M. Barreiro, K. K. Hii, Chem.Euro. J., 2012, 18, 11317–11324.
[8]: Gaussian Job Archive for C21H28O7. Henry S. Rzepa. figshare. http://dx.doi.org/10.6084/m9.figshare.738037.
[9]: Schoeberl, Christof; Jaeger, Volker Advanced Synthesis and Catalysis, 2012 , vol. 354, # 5 p. 790 - 796
[10]: Forbes, David C.; Bettigeri, Sampada V.; Patrawala, Samit A.; Pischek, Susanna C.; Standen, Michael C. Tetrahedron, 2009 , vol. 65, # 1 p. 70 - 76
[11]: Schoeberl, Christof; Jaeger, Volker Advanced Synthesis and Catalysis, 2012 , vol. 354, # 5 p. 790 - 796
[12]: Reusch; Johnson Journal of Organic Chemistry, 1963 , vol. 28, p. 2557
