Rep:Mod:YTL14
Introduction [1]
A potential energy surface (PES) is used to analyse the molecular structure change in a chemical reaction. In a simple diatomic molecule, the 1D PES is a curve resulting from the combination of functions considering (1) the repulsion between the two atoms at very short internuclear distance (2) Coulombic attraction between the two ions and (3) energy at equilibrium is represented by a Harmonic oscillator. This provide a curve with energy at the y-axis and internuclear distance or the reaction coordinate at the x-axis. This estimation is good for simple diatomic molecule formation/dissociation.
However, as the molecule becomes larger more variables are involved. The degrees of freedom (3N-6 for non-linear structure, while 3N-5 for linear molecule; N is the number of atom) gives the dimensionality of the PES. In the determination of degrees of freedom, the translational and rotational energy of the molecule are opt out as the potential energy of a molecule does not change upon translation or rotation in space. PES is dependent on the relative position of the one atom to another (geometry).
A minima on PES is a point corresponds to either transition states, reactants, products and intermediates. It can be classified as local minima or global minima. A saddle point on a PES is the maximum connecting two minima in the minimum energy pathway, i.e the transition state. As at this point going down either direction will result in a lower energy conformation/structure. The gradient and thus the force at the transition state is zero. The same applies to the minima. These points at which the forces are zero also known as stationary point. A frequency calculation of the stationary point allows one to understand the nature of the structure at that particular point such as frequency, vibrational modes etc. The transition state is the first saddle point by definition and is indicated by the presence of one negative frequency (imaginary frequency).
Nf710 (talk) 17:46, 21 March 2017 (UTC) I can see that you have a very good understanding here, however you should have explained this with second derivatives.
Exercise 1: Reaction of Butadiene with Ethylene
Method 2 was used. The reactants, transition state and product are optimised at PM6 level.
Diels-Alder reaction is thermal cycloaddition between a conjugated, electron-rich diene and an electron-poor dienophile. The conjugated diene must adopt a s-cis conformation. The interaction between the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) of the reactants participate in bond formation. As electron density 'flows' (or charge transfer) from HOMO to LUMO.
| Species | HOMO | LUMO | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|||||||||
|
| Species | MO | ||||||
|
Interaction between HOMO of ethene and LUMO of butadiene | ||||||
|
| ||||||
| Interaction between LUMO of ethene and HOMO of butadiene | |||||||
|
| ||||||
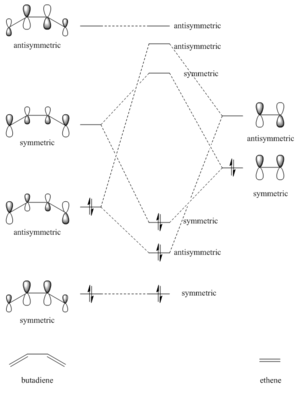
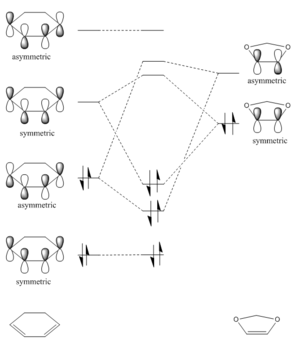
From the transition state MOs, it is clearly shown that the interacting MOs are as follow:
1. HOMO (butadiene) - LUMO (ethene) give HOMO-1 and LUMO+1. All four MOs are asymmetric. 2. LUMO (butadiene) - HOMO (ethene) give HOMO and LUMO. All four MOs are symmetric.
Hence it is confirmed that for an allowed interaction, the HOMO and LUMO have to be of the same symmetry. While for the case where the interacting MOs are of different symmetry the reaction is forbidden.
The two bonding MOs generate the two new C-C σ-bonds in 1-cyclohexene.
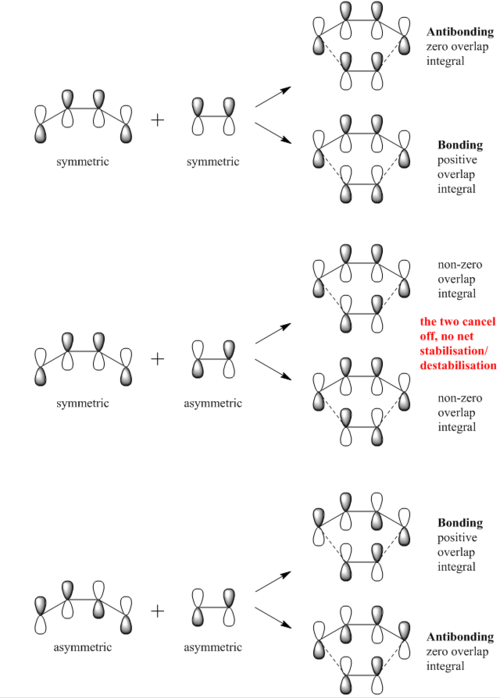
(Fv611 (talk) 17:31, 15 March 2017 (UTC) While your MO diagram in Figure 2 is correct, there is a lot of confusion on the transition state MOs in Figure 3. I think you are forgetting that we are talking about orbital overlap, not electron density overlap. Both bonding and antibonding interactions will yield a non-zero orbital overlap if they arise from fragment orbitals with the same symmetry (S/S or AS/AS). On the other hand, S/AS interactions are not allowed because they result in a zero orbital overlap. Additionally, you wrongly assigned antibonding and bonding to the MOs arising from S/S combinations in Figure 3 (the top transition state MO is the bonding orbital). Finally, the correct term is antisymmetric, not symmetric.)
| Reactants | Product | Vibrations at transition state | ||
|---|---|---|---|---|
 |
 |
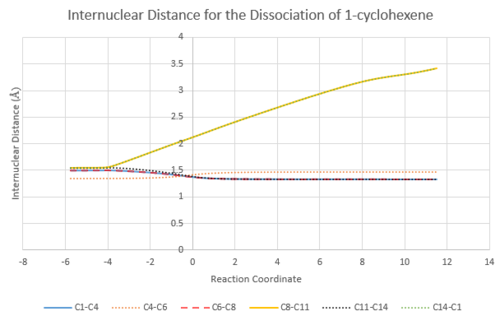
IRC showed the dissociation of 1-cyclohexene to butadiene and ethene. Referring to Figure 4, reaction coordinates at -5.73, 0 and 11.49 correspond to product, transition state and reactants respectively.
Two sp3 C-C bonds are formed between C8 and C11, and C14 and C1. New sp2 C=C bond is formed between C4 and C6. The sp2 C=C and sp3 C-C bond lengths are of good comparison to literature (1.36 Å and 1.54 Å respectively). The Van der Waals radius of a carbon atom is 1.70 Å. In the transition state, the distance between the carbons forming new bonds are 2.115 Å, which is in between two times the Van der Waals radius (3.40 Å) and 1.70 Å. [2]
| Bond broken | |||||
| Reactant | Bond length (Å) | Transition state | Bond length (Å) | Product | Bond length (Å) |
| C11=C14 | 1.327 | C11--C14 | 1.382 | C11-C14 | 1.541 |
| C1=C4 | 1.335 | C1--C4 | 1.38 | C1-C4 | 1.501 |
| C6=C8 | 1.335 | C6--C8 | 1.38 | C6-C8 | 1.501 |
| Bond formed | |||||
| Reactant | Bond length (Å) | Transition state | Bond length (Å) | Product | Bond length (Å) |
| C4-C6 | 1.468 | C4--C6 | 1.411 | C4=C6 | 1.338 |
| C8,C11 | non-bonding | C8--C11 | 2.115 | C8-C11 | 1.54 |
| C14,C1 | non-bonding | C14--C1 | 2.115 | C14-C1 | 1.54 |
TS bond lengths resemble the reactants' bond length (compare to products'), hence according to Hammond's postulate this is an early transition state.
Formation of the two bonds is synchronous, i.e concerted, with the same bond lengths. No radical nor ionic intermediates was formed.
Exercise 2: Reaction of Cyclohexadiene and 1,3-Dioxole
In this exercise, Diels-Alder reaction of cyclohexadiene and 1,3-Dioxole were studied. The reactants, transition states and products were optimised at PM6 level. The transition states were reoptimised at B3LYP-6d to confirm the structure obtained. Thermochemistry study was performed using the IRC data obtained at PM6 level.
| Species | HOMO | LUMO | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
| |||||||||
|
|
|
The HOMO of cyclohexadiene and LUMO of 1,3-dioxole are both asymmetric, hence they interact to form LUMO+1 and HOMO-1 orbitals in the transition states. Meanwhile, the LUMO of cyclohexadiene and HOMO of 1,3-dioxole are both symmetric. The interaction of both results in HOMO and LUMO orbitals in the transition states.
(Use antisymmetric. Asymmetric means a lack of symmetry and antisymmetric means the sign/phase is changed when the symmetry operation is performed Tam10 (talk) 16:31, 15 March 2017 (UTC))
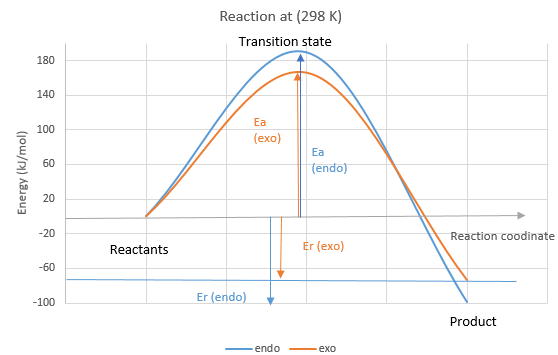
(Avoid placing different IRCs on the same coordinate. Energies of stationary points such as reactants, TS and products can be compared this way, but the x axis of an IRC is specific Tam10 (talk) 16:31, 15 March 2017 (UTC))
The endo product is both the thermodynamic (more negative reaction energies (Er), indicative of a higher stability) and kinetic product (lower activation energy (Ea), hence the endo product forms faster). Hence endo product is the major product.
Nf710 (talk) 18:05, 21 March 2017 (UTC) Thats is not what your graph is showing?
Diels-Alder reaction between cyclohexadiene and 1,3-Dioxole are inverse electron demand. The 1,3-dioxole is electron rich due to the delocalisation of oxygen lone pair into the pi-system of 1,3-dioxole. This creates a repulsion of the donated electron and HOMO electrons. This results in a rise in HOMO energy, which then has a comparable energy with the LUMO of the diene. The LUMO of 1,3-dioxole and HOMO of cyclohexadiene are closer in energy. Hence they interact more strongly than HOMO of 1,3-dioxole and LUMO of cyclohexadiene.(HOMO-diene and LUMO-dienophile interact stronger in normal oxygen demand Diels-Alder).
| Energy | 0 K | 298 K | ||
| Endo | Exo | Endo | Exo | |
| Reactant (Hartree/particle) | 0.120615 | 0.118665 | 0.065793 | 0.075809 |
| Transition state (Hartree/particle) | 0.172488 | 0.173266 | 0.137941 | 0.138906 |
| Product (Hartree/particle) | 0.070675 | 0.070929 | 0.037802 | 0.037976 |
| Activation energy (kJ/mol) | 136.1926 | 143.3549 | 189.4351 | 165.6612 |
| Reaction energy (kJ/mol) | -131.117 | -125.3309 | -99.33054 | -73.4799 |
In Diels-Alder reaction, the endo pathway is normally the preferred pathway due to secondary interaction which is said to lower the energy of the transition state.
Oxygen is marked red.
Nf710 (talk) 18:10, 21 March 2017 (UTC) You have done your calculations at PM6 and therefore got the wrong answers. You should have used B3LYP You have also contradicted yourself with the graph.
Exercise 3: Hetero-Diels-Alder and Cheletropic of o-xylylene and SO2
The Hetero-Diels-Alder and Cheletropic reaction between o-xylylene and SO2 were studied. The products of these reactions were firstly optimised, the newly formed bonds on the optimised product were broken and followed by optimization of the distorted structure. IRC calculation was performed on the transition states obtained. The reactants and products from the IRC were re-optimized. All calculations were done at PM6 level.
| Energy | 0 K | 298 K | ||||
| Endo | Exo | Cheletropic | Endo | Exo | Cheletropic | |
| Reactant (Hartree/particle) | 0.116934 | 0.116955 | 0.114807 | 0.058769 | 0.059656 | 0.070991 |
| Transition state (Hartree/particle) | 0.126589 | 0.128172 | 0.13556 | 0.090559 | 0.092082 | 0.099062 |
| Product (Hartree/particle) | 0.057503 | 0.056644 | 0.034556 | 0.021704 | 0.021455 | 0.0000002 |
| Activation energy (kJ/mol) | 25.34395 | 29.45023 | 52.43245 | 83.45939 | 85.13446 | 70.92138 |
| Reaction energy (kJ/mol) | -156.0413 | -158.3465 | -70.92138 | -97.31941 | -100.2967 | -179.3638 |
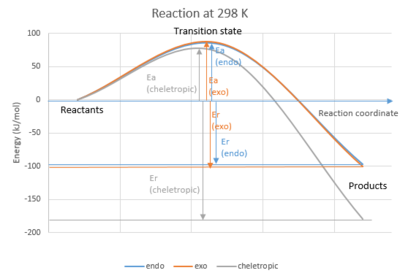
(We asked that you used the sum of the energies of xylylene and SO2 for consistency Tam10 (talk) 16:31, 15 March 2017 (UTC))
From Table 8 and Figure 7, it was found that cheletropic product was the thermodynamic and kinetic product.
| Reaction | Transition state | Product | IRC | ||||
|---|---|---|---|---|---|---|---|
| Diels Alder (Endo) |  | ||||||
| Diels Alder (Exo) |  | ||||||
| Cheletropic |  |
Diels-Alder and cheletropic products all contain an aromatic benzyl group. At the beginning of the reaction, the six-membered ring in xylylene has 2 C=C bonds. As the new bonds begin to form, all the six bonds are in conjugation (C-C bond is in between C-C single bond and C=C bond). The products formed are aromatic with pi-orbital above and below the plane. Xylylene is unstable compared to the product. Aromaticity is the driving force of the reaction.
(Fairly short write-up for this section Tam10 (talk) 16:31, 15 March 2017 (UTC))
Reference
- ↑ http://www.uniheidelberg.de/institute/fak12/AC/hofmann/acf_theo/MinNoMin.pdf
- ↑ S. S. Batsanov, Inorg. Mater. Transl. from Neorg. Mater. Orig. Russ. Text, 2001, 37, 871–885.