Rep:Mod:Y3CPsfl10
Year 3 Computational Lab: Physical Experiment
The protocol followed in this exercise can be found here.
The Cope Rearrangement Tutorial
1,5-hexadiene Optimisations
Anti Conformers
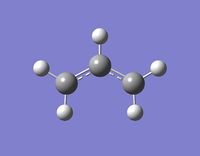
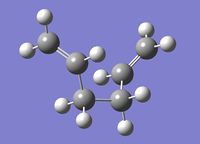
An anti 1,5-hexadiene molecule was optimised to a minimum at the HF/3-21G level of theory. This particular anti conformer (antiA) was found to be equivalent to anti1 in the table here. Anti1 in this table has an energy of -231.69260 a.u., which is very close to antiA's energy: -231.69260235 a.u.. They both have a point group C2, and look the same. Figure 1 (right) is a picture of antiA, and its log file can be found here
Gauche Conformers
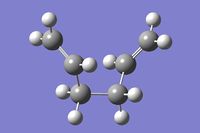
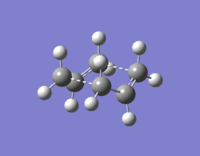
A gauche 1,5-hexadiene molecule was optimised to a minimum at the HF/3-21G level of theory. This particular gauche conformer (gaucheA) was found to be equivalent to gauche2 in the table here. Gauche2 in this table has an energy of -231.69167 a.u., which is almost the same as gaucheA, with a value of -231.69166 a.u.. This gaucheA structure and gauche2 have the same point group, C2. Figure 2 shows of gaucheA, and its log file can be found here
Finding the Lowest Energy Conformer
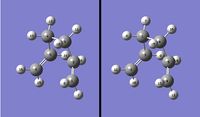
An attempt to find the lowest energy conformer was made by taking antiA (figure 1), chosen due to it being lower in energy than guacheA, and making the bond angles larger to reduce any steric effects. The structure was again optimised to a minimum at the HF/3-21G level. A picture of this molecule is shown in figure 3. The tweaks to the structure made little difference, but was very slightly lower, the new molecule having an energy of -231.69260237 a.u..
Further attempts to tweak the structure yielded a structure which was closer in energy to anti2, and so higher in energy than my original anti1 structure; energy of optimised structure (HF/3-21G optimisation to a minimum): -231.69253528 a.u., point group: C1. This point group is not the same as that of anti2, which is investigated in the section below. The only anti conformer listed in the table with a C1 symmetry is anti4, which looks nothing like my molecule, and has the significantly higher energy of -231.69097 a.u..
The anti2 Conformer
The attempt at drawing and optimising the anti2 structure as seen here was successful, giving a molecule of energy -231.69253527 a.u., which correlates well with the value given in the table of -231.69254 a.u.. Symmetrising the molecule gave a Ci point group, the same as that of the molecule in the table.
The anti2 molecule was then further optimised to a minimum at the B3YLP/6-31G* level, giving a significantly lower energy of -234.61170290 a.u., the point group remaining Ci. A picture of this reoptimised molecule is shown in figure 4, and its log file is available here.
Table 1 (below) shows all relevant energies associated with this molecule (from both levels of optimisation).
| All units in hartree | HF/3-21G | B3LYP/6-31G* | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Electronic energy | Sum of electronic and zero-point energies | Sum of electronic and thermal energies | Sum of electronic and thermal enthalpies | Sum of electronic and thermal free energies (298.15 K) | Electronic energy | Sum of electronic and zero-point energies | Sum of electronic and thermal energies | Sum of electronic and thermal enthalpies | Sum of electronic and thermal free energies (298.15 K) | |
| anti2 from table | -231.692535 | -231.539539 | -231.532566 | -234.611710 | -234.469203 | -234.461856 | ||||
| Calculated anti2 | -231.69253527 | -231.436741 | -231.430933 | -231.466250 | -231.466250 | -234.61170290 | -234.469204 | -234.461857 | -234.460913 | -234.500778 |
Table 1: Energies associated with the anti2 conformer; comparing previously reported data with new
Optimizing the Chair and Boat Transition States
Chair
An allyl fragment C3H5 was optimised to the HF/3-21G level of theory, giving a molecule of energy -115.82304010 a.u., which can be seen in figure 5 and who's log file can be found here. Using 2 units of this fragment, a chair transition state was guessed and optimised to a transition state using TSBerny to the HF/3-21G level of theory (chair optimisation A). This gave rise to a structure with an imaginary vibration of -817.9cm-1 and energy of -231.61932230 a.u.. This imaginary vibration can be seen in figure 6, and it's log file can be found here. After this optimisation the bond making/breaking distances had dropped to 2.02Å.
The original (unoptimised) chair transition structure was then optimised to a minimum at the HF/3-21G level, freezing the 4 atoms involved in bond breaking/making (chair optimisation B). This gave a molecule of energy -231.61504992 a.u., which was then further optimised as a transition state with TSBerny to the HF/3-21G level (chair optimisation C), giving a molecule of energy -231.61932245 a.u. and imaginary frequency -817.96 cm-1, the log file for this final optimised structure can be found here.
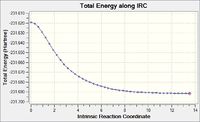
To find what structure a chair transition state is likely to drop to, an IRC was run forwards on this final optimised structure (from optimisation C), looking at 50 points, force constants being calculated all along the way at the HF/3-21G level of theory. The calculation stopped at point 44, giving the molecule shown in figure 7, of energy -231.69157878 a.u. and an RMS gradient of 0.00015223 a.u., log file available here. A graph of the total energies from this IRC can be seen in figure 8, clearly showing point 44 at a minimum. This molecule was then optimised to a minimum at the HF/3-21G level (chair optimisation D) to give a molecule of energy -231.69166702 a.u. and an RMS gradient of 0.00000475 a.u., log file: here.
An IRC was also run on our first optimised structure (from optimisation A) again looking at 50 points at the HF/3-21G level of theory. The calculation again stopped, this time at a structure of energy -231.69157869 a.u. at an RMS gradient of 0.00015227 a.u.. When this minimum was then optimised to a minimum at the HF/3-21G level (chair optimisation E), the same molecule as from optimisation D above was obtained, with energy: -231.69166702 a.u., point group C2 and RMS gradient 0.00000475 a.u.. This final molecule can be seen in figure 9, and is equivalent to gauche2 in this table, being of the same energy, point group and conformation.
Boat
To obtain a structure for a boat transition state, the previously optimised anti2 structure was used as a skelton. The carbons were numbered appropriately and a TS(QST2) optimisation was run on the structures to find the Cope rearrangement (figure 10) transition state, which should be a boat. Initially this did not work as the anti2 structure had been left linear. The program could therefore not line up the appropriate orbitals and the calculation failed. The structures were therefore altered so that the atoms were correctly aligned for a Cope rearrangement (see figure 11). Using the atom labels in figure 11, the bond angles were changed to the following angles for the reactant molecule: C2-C3-C4-C5 to 0o and C2-C3-C4 & C3-C4-C5 to 100o. The product molecule's bond angles were similarly changed to: 0o for C2-C1-C6-C5 and 100o for C2-C1-C6 & C1-C6-C5. This worked well, and a boat transition structure was calculated. The boat had an energy of -231.60280168 a.u. and an imaginary frequency of -837.67cm-1. This vibration can bee seen in figure 12.
Activation energies for boat and chair conformers
The chair structure from chair optimisation C was further optimised to a transition state to the B3YLP/6-31G* level of theory, to give a molecule of energy -234.55698303 a.u. with an imaginary frequency of -565.54 cm-1. The boat structure obtained from the QST2 calculation was further optimised to a transition state to the B3YLP/6-31G* level, giving a molecule of energy -234.54309307 a.u., with an imaginary vibration of -530.37 cm-1
| HF/3-21G | B3LYP/6-31G* | ||||||
|---|---|---|---|---|---|---|---|
| Electronic energy | Sum of electronic and zero-point energies | Sum of electronic and thermal energies | Electronic energy | Sum of electronic and zero-point energies | Sum of electronic and thermal energies | Units | |
| at 0 K | at 298.15 K | at 0 K | at 298.15 K | ||||
| Chair TS | -231.61932245 | -231.466701 | -231.495207 | -234.55698303 | -234.414929 | -234.443814 | hartree |
| Reactant (gauche2) | -231.69166701 | -231.538704 | -231.570133 | -234.61068811 | -234.468263 | -234.499305 | hartree |
| ΔE gauche2 -> chair | 0.07234456 | 0.072003 | 0.074926 | 0.05370508 | 0.053334 | 0.0579236 | hartree |
| Boat TS | -231.60280168 | -231.450928 | -231.479774 | -234.54309307 | -234.402342 | -234.431751 | hartree |
| Reactant (anti2) | -231.69253527 | -231.436741 | -231.466250 | -234.61170290 | -234.469204 | -234.500778 | hartree |
| ΔE anti2 -> boat | 0.08973359 | 0.014187 | 0.013524 | 0.06860983 | 0.066862 | 0.069027 | hartree |
The Diels Alder Cycloaddition
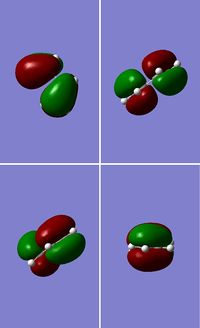
Optimisation of cis-Butadiene
A molecule of cis-butadiene was drawn out, cleaned and optimised using the AM1 semi-empirical method, giving a molecule of energy 0.04878534 a.u. The HOMO and LUMO were visulalised, and can be seen in figures 13 and 14 respectively. The pictures in the first row of both figures cleary show the nodal planes of the two MOs, the HOMO having 2 and the LUMO having 3. The HOMO is clearly antisymmetric, whereas the LUMO is symmetric.
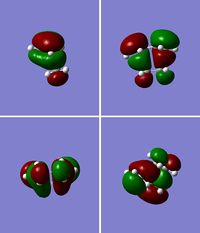
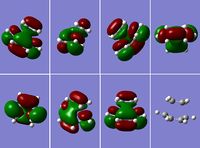
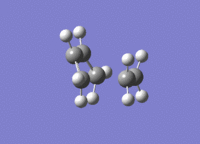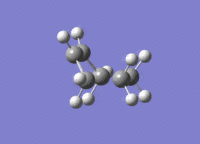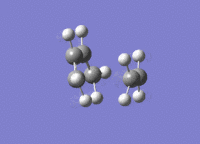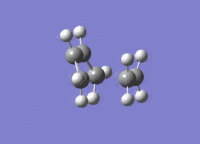
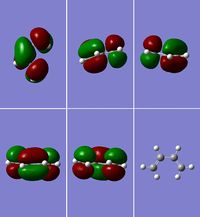
Transition State of "Prototype" Reaction
Using the previously optimised structure of cis-butadiene, the transition state for a Diels-Alder cycloaddition between cis-butadiene and ethylene was drawn out and optimised as a transition state with TSBerny at the semi-empirical AM1 level. The top right and bottom left pictures in figure 15 clearly show the 3 nodal planes of the HOMO of this transition state, whilst the nodal planes of the LUMO are much less clearly defined (figure 16); there are 2 nodal planes and 1 nodal curved surface which can be clearly seen in the picture of the second row, second from the left. Again, the HOMO is antisymmetric and the LUMO is symmetric. The energy for this transiton state is 0.11165465 a.u., it has an imaginary frequency of: -956.17 cm-1 and a point group of C1. The imaginary frequency can be seen in figure 17.
Studying the Regioselectivity of the Diels Alder Reaction
In this section we will investigate the Diels Alder reaction of cyclohexa-1,3-diene with maleic anhydride. The primary structure formed will be the endo adduct has the endo transition state is lower energy, but we will also invesigate the exo transition state.
Optimising the reactants
Semi-empirical AM1 optimisation
Cyclohexa-1,3-diene was drawn out and optimised at the semi-empirical AM1 level of theory, giving a molecule of energy 0.02771129 a.u.. Maleic anhydride was drawn out and optimised to the semi-empirical AM1 level aswell, giving a molecule of energy -0.12182418 a.u..
HF/3-21G & B3YLP/6-31G* optimisations
Cyclohexa-1,3-diene was drawn out and optimised to a minimum to the HF/3-21G level of theory, giving a molecule of energy -230.54323108 a.u.. This was then further optimised to a minimum to the B3YLP/6-31G* level, giving a molecule of energy -233.41893632 a.u.. Maleic anhydride was also optimised to a minimum twice; to HF/3-21G and then further to B3YLP/6-31G*, giving molecules of energy -375.10351323 a.u. and -379.28954468 a.u. respectively.
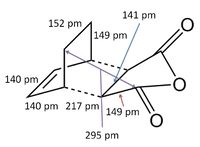
Transition State Energies
3 Exo
Combining the structures from the semi-empirical AM1 optimisations
The structures previously optimised to AM1 were combined to give a transition state for an exo Diels Alder reaction. These were then optimised to a transition state with TSBerny to AM1, giving a transition state of energy -0.05041975 a.u. with an imaginary frequency of -812.67 cm-1. This transition state can be seen in figure 18. The bond lengths of the C-C distances are shown in figure 19.
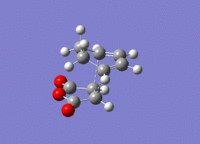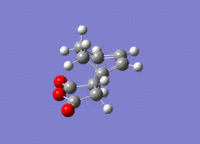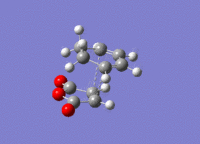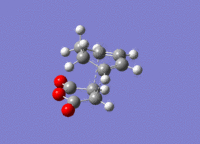
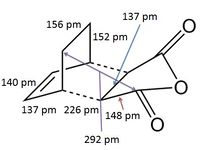
Combining the structures from the HF/3-21G & B3YLP/6-31G* optimisations
The structures previously optimised to the B3YLP/6-31G* level were combined to give a transition state for an exo Diels Alder reaction. These were then optimised to a transition state with TSBerny to HF/3-21G, giving a transition state of energy -605.60359124 a.u. with an imaginary frequency of -647.36 cm-1. This transition state can be seen in figure 20.
A structure for the exo product was optimised to HF/3-21G level, giving a molecule of energy -605.71873542 a.u.. The two previously optimised (to B3YLP/6-31G*) reactants were placed in the same file in an exo orientation and a QST2 calculation was run to HF/3-21G to find the transition state, to compare with the guess structure shown in figure 20. The transition state from this calculation had an energy of -605.60359125 a.u. and an imaginary frequency of -647.48 cm-1. Both of these values are close to the values determined by my guess structure, which would suggest that the guess was quite good. The bond lengths from this transition state can be seen in figure 21.
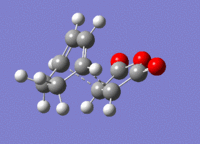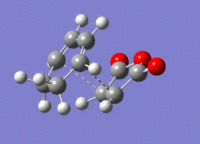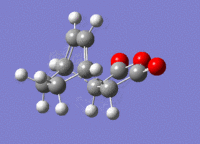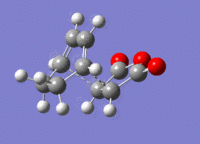
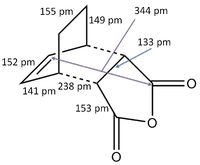
4 Endo
Combining the structures from the semi-empirical AM1 optimisations
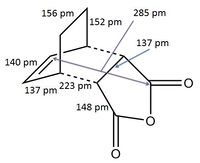
The structures previously optimised to AM1 were combined to give a transition state for an endo Diels Alder reaction. These were then optimised to a transition state with TSBerny to AM1, giving a transition state of energy -0.05150474 a.u.. with an imaginary frequency of -806.40 cm-1. This transition state can be seen in figure 22. The bond lengths of the C-C distances are shown in figure 23.
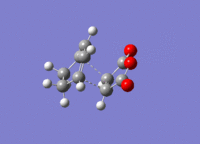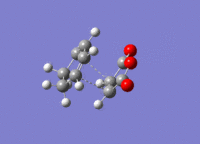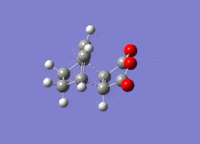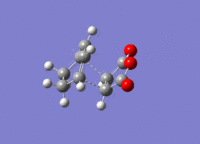
Combining the structures from the HF/3-21G & B3YLP/6-31G* optimisations
The structures previously optimised to the B3YLP/6-31G* level were combined to give a transition state for an endo Diels Alder reaction. These were then optimised to a transition state with TSBerny to HF/3-21G, giving a transition state of energy -605.61036822 a.u. with an imaginary frequency of -643.58 cm-1. This transition state can be seen in figure 24.
A structure for the endo product was optimised to HF/3-21G level, giving a molecule of energy 605.72132083 a.u.. The two previously optimised (to B3YLP/6-31G*) reactants were placed in the same file in an endo orientation and a QST2 calculation was run to HF/3-21G to find the transition state, to compare with the guess structure shown in figure 22. The transition state from this calculation had an energy of -605.61036811 a.u. and an imaginary frequency of -643.19 cm-1. Both of these values are close to the values determined by my guess structure, which would suggest that the guess was quite good. The bond lengths from this transition state can be seen in figure 25.
References
The protocol followed in this exercise can be found here.
