Rep:Mod:XYZ12346
Inorganic Module 2
This week is all about learning how to operate Gaussian , optimising structure of simple molecules and measuring properties such as IR frequencies, charge distribution and molecular orbitals.In the first part of the lab optimisation of 3 molecules was made , using different basis set and pseudo-potential to obtain accurate calculations for the molecules in question.Pseudo-potential needs to be used to make calculations for heavy , many electrons atoms such as thallium, as it cant be solved by the standard Schrodinger equation.This method is possible because of the assumption that valence electrons contribute mostly to bonding. When a molecule contains a mixture of light and heavy atoms , we need to use a combination of pseudo-potential and basis set.
WEEK 1
Optimisation
| Molecule | Bond distance(A) | Bond distance(A) lit. | Bond angles |
|---|---|---|---|
| BH3 | 1.19 | 1.191 | 120.0 |
| BBr3 | 1.93 | 1.891 | 120.0 |
| TlBr3 | 2.65 | 2.621 | 120.0 |
The bond length increases as hydrogen atoms are changed to bromine atoms.The same applies for changing the central element ,however the change in bond length is greater in this case.So as follows Tl-Br would be the longest bond , followed by B-Br and the shortest bond would be B-H.This is due to increase in the size of the substituted ligands(bigger mass and larger amount of electrons), the atomic radius increases i.e electronic cloud around the nucleus is more spread from the centre and a favorable electron interaction occurs at longer distance.Tl is much more electron rich than B and Br is more electron rich than H , also H posses only one 1s orbital for bonding , however Br can use p orbitals to bond to the center element. Electrons in the inner shells of thallium can influence the valence electrons and therefore influence the bonding, its s orbitals are too deep down in energy for bonding.Both B and Tl can use p orbitals for bonding. The bond angle and symmetry remains the same in all 3 molecules. The values are in a reasonable close agreement to the literature values which indicates that the program aproximates the ground state of molecule quite well.
Reference 1. CRC book of chemistry and physics, 92 edition
Summary
- BH3 Optimisation
| File name | bh3_opt |
| File type | .log |
| Calculation type | FOPT |
| Calculation method | RB3LYP |
| Basis set | 3-21G |
| Charge | 0 |
| Spin | Singlet |
| E(RB3LYP) | -26.46226433 a.u. |
| RMS Gradient Norm | 0.00004507 a.u. |
| Imaginary Freq | |
| Dipole Moment | 0.00 Debye |
| Point Group | D3H |
| Job cpu time | 0 days 0 hours 0 minutes 3.0 seconds. |
Item Value Threshold Converged?
Maximum Force 0.000090 0.000450 YES
RMS Force 0.000059 0.000300 YES
Maximum Displacement 0.000352 0.001800 YES
RMS Displacement 0.000230 0.001200 YES
Predicted change in Energy=-4.580970D-08
Optimization completed.
-- Stationary point found.
| File name | BH3_OPT_631g_1_TRIAL 2 |
| File type | .log |
| Calculation type | FOPT |
| Calculation method | RB3LYP |
| Basis set | 6-31G(d,p) |
| Charge | 0 |
| Spin | Singlet |
| E(RB3LYP) | -26.61532363 a.u. |
| RMS Gradient Norm | 0.00000291 a.u. |
| Imaginary Freq | |
| Dipole Moment | 0.00 Debye |
| Point Group | D3H |
| Job cpu time | 0 days 0 hours 0 minutes 3.0 seconds. |
Item Value Threshold Converged?
Maximum Force 0.000006 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000023 0.001800 YES
RMS Displacement 0.000015 0.001200 YES
Predicted change in Energy=-2.012162D-10
Optimization completed.
-- Stationary point found.
- Links
Optimisation
here here
- TlBr3
| File name | TlBr3_log_HTS |
| File type | .log |
| Calculation type | FOPT |
| Calculation method | RB3LYP |
| Basis set | LANL2DZ |
| Charge | 0 |
| Spin | Singlet |
| E(RB3LYP) | -91.21812851 |
| RMS Gradient Norm | 0.00000088 |
| Imaginary Freq | |
| Dipole Moment | 0.00 Debye |
| Point Group | D3H |
| Job cpu time | 0 days 0 hours 0 minutes 15.4 seconds. |
Item Value Threshold Converged?
Maximum Force 0.000002 0.000450 YES
RMS Force 0.000001 0.000300 YES
Maximum Displacement 0.000016 0.001800 YES
RMS Displacement 0.000010 0.001200 YES
Predicted change in Energy=-4.107348D-11
Optimization completed.
-- Stationary point found.
- Links
Optimisation
DOI:10042/22051
- BBr3 Optimisation
| File name | BBr3_OPT_0_Gen |
| File type | .log |
| Calculation type | FOPT |
| Calculation method | RB3LYP |
| Basis set | Gen |
| Charge | 0 |
| Spin | Singlet |
| E(RB3LYP) | -64.43645296 a.u. |
| RMS Gradient Norm | 0.00000382 a.u. |
| Imaginary Freq | |
| Dipole Moment | 0.00 Debye |
| Point Group | D3H |
| Job cpu time | 0 days 0 hours 0 minutes 8.0 seconds. |
Item Value Threshold Converged?
Maximum Force 0.000008 0.000450 YES
RMS Force 0.000005 0.000300 YES
Maximum Displacement 0.000036 0.001800 YES
RMS Displacement 0.000023 0.001200 YES
Predicted change in Energy=-4.027534D-10
Optimization completed.
-- Stationary point found.
- Links
Optimisation
here
Vibrational analysis
| frequency | Intensity | Symmetry D3h point group | |||
|---|---|---|---|---|---|
| BH3 | TlBr3 | BH3 | TlBr3 | BH3 | TlBr3 |
| 1163 | 46 | 94 | 4 | A'2 | E' |
| 1213 | 46 | 14 | 4 | E' | E' |
| 1213 | 52 | 14 | 6 | E' | A'2 |
| 2582 | 165 | 0 | 0 | A'1 | A'1 |
| 2715 | 211 | 126 | 25 | E' | E' |
| 2715 | 211 | 126 | 25 | E' | E' |
Itcan be clearly seen that frequencies are much lower for Tl molecule, this is an indication of the big difference in mass between B and Tl and H and Br, as it can be predicted , heavier atoms would vibrate more slow , and the process would require more energy as the bonds are also longer.That would also affect the intensity of the vibration which is also lowered due to this reason.There has also been a reordering of modes(A'2 and E' symmetry frequencies have switched places) Low frequencies are not shown in the table because they represent small motions of the central atom , which don't include bond stretching or bending. For example the first normal mode vibration for TlBr3 is degenerate bends at frequency 46.43 cm-1 , which is a bend of two Tl-Br bonds towards each other and a bend of another Tl-Br ,coupled with the vibrations of metal centre.Looking at the theoretical IR spectra it can be seen that for both molecules there are only 3 peaks ,because the vibration one vibration in each is IR inactive, as the vibrations are completely symmetric and therefore the molecule doesn't possess a dipole(stretch with A'2 symmetry). Two vibrations(no.2 and no.3)at 1213 cm-1 and (no.1 and no.3) at 46.4 cm-1 are degenerate and therefore appear at the same energy. This also applies to two vibrations at 2715cm-1 and 126.3 cm-1. For both molecules one of those set of frequencies lies lower in energy and another much higher in energy, this is linked to the nature or the motion. Asymmetric stretches would require more energy to perform compared to the bond bends which would be require less energy.It can also be seen that the difference between 2 sets of degenerate frequencies is much higher in BH3 Frequency analysis has to be made to make sure the optimisation has given us the structure of a molecule which is as close as possible to the ground state molecule , as the molecule in the ground state would be in equilibrium with the forces acting on it(bonding , intermolecular interactions etc.) and therefore would have low frequencies(tending to 0) of the motion of the centre atom , as there is no forces making it to move.Also performing a frequency analysis means finding a second derivative of the potential energy surface , and therefore if all the frequencies are positive it means we archived a minimum energy state. Method determines the approximations that are taken when solving the Schrodinger equation for each positions of atoms , and the basis set determines the accuracy, If two different sets are used for optimisation and frequency analysis it would bring inconsistency into the results , cause the problem might be probing slightly different structures and at different degree of accuracy.
Low frequencies
- BH3
Low frequencies --- -0.9432 -0.8611 -0.0054 5.7455 11.7246 11.7625 Low frequencies --- 1162.9963 1213.1826 1213.1853
Item Value Threshold Converged?
Maximum Force 0.000006 0.000450 YES
RMS Force 0.000003 0.000300 YES
Maximum Displacement 0.000023 0.001800 YES
RMS Displacement 0.000011 0.001200 YES
Predicted change in Energy=-1.996522D-10
Optimization completed.
-- Stationary point found.
Frequency
here
- TlBr3
Low frequencies --- -3.4213 -0.0026 -0.0004 0.0015 3.9367 3.9367 Low frequencies --- 46.4289 46.4292 52.1449
Item Value Threshold Converged?
Maximum Force 0.000002 0.000450 YES
RMS Force 0.000001 0.000300 YES
Maximum Displacement 0.000022 0.001800 YES
RMS Displacement 0.000011 0.001200 YES
Predicted change in Energy=-5.660901D-11
Optimization completed.
-- Stationary point found.
Frequency
DOI:10042/22052
IR spectrum
MO analysis
MO diagram
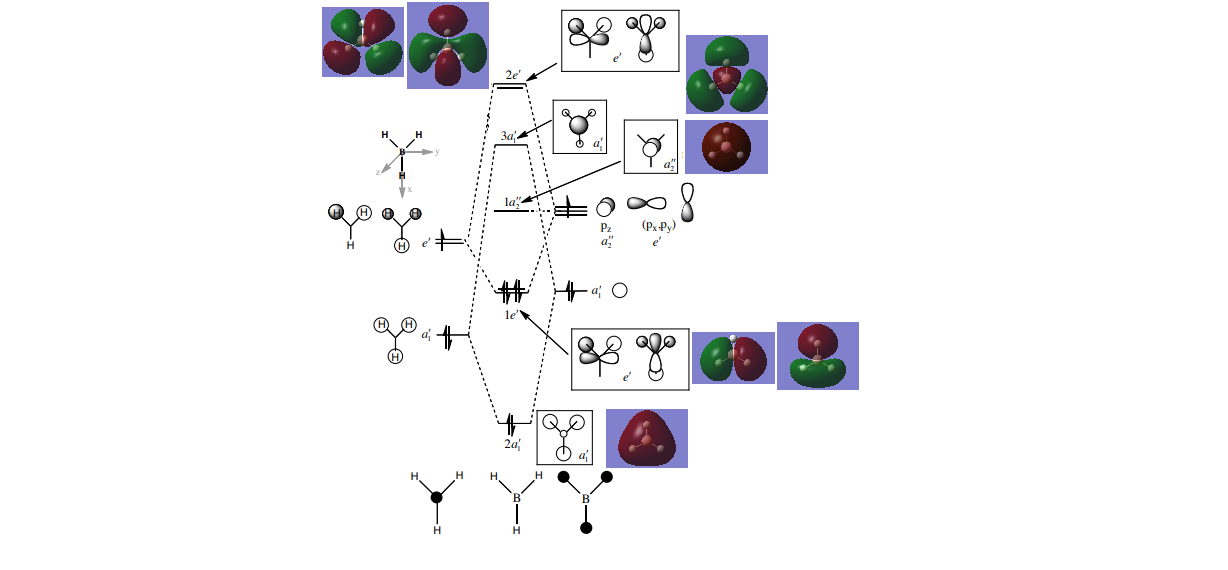
As can be seen from the diagram for a simple system like BH3 with a small amount of electrons on each atom LCAO MO diagram is in good agreement with the computed MO.Therefore it is a useful tool and makes us think about possible combinations of the AOs and their relative energies. It can be seen that real MOs are more delocalised around the molecule, and when more orbitals participate in the bonding , their contributions can be seen more clearly. Also LCAO predicts that the highest energy level(a'1) would correlate to the antibonding MO coming form the overlap of 2s(B) and three out of phase 1s (H) orbitals, however when NBO analysis was performed , the e' degenerate levels have been shown as the highest energy MOs. This is an example of a limitation of LCAO MO as the splitting of orbitals can be only approximated and therefore the ordering of the predicted levels might be different. It is also easier to compute the relative sideways bonding/anti-bonding character of the MOs as it is up to a subjective opinion in the case of LCAO MO. The lowest MO , corresponds to a non bonding 1s orbital of boron , it is too low in energy for bonding.
Link
here
NBO analysis
Optimisation
- NH3
| File name | NH3_OPTIMISATION_TRIAL2 |
| File type | .log |
| Calculation type | FOPT |
| Calculation method | RB3LYP |
| Basis set | 6-31G(d,p) |
| Charge | 0 |
| Spin | Singlet |
| E(RB3LYP) | -56.55776863 a.u. |
| RMS Gradient Norm | 0.00000289 a.u. |
| Imaginary Freq | |
| Dipole Moment | 1.85 Debye |
| Point Group | C3V |
| Job cpu time | 0 days 0 hours 0 minutes 11.0 seconds. |
Item Value Threshold Converged?
Maximum Force 0.000005 0.000450 YES
RMS Force 0.000003 0.000300 YES
Maximum Displacement 0.000010 0.001800 YES
RMS Displacement 0.000007 0.001200 YES
Predicted change in Energy=-7.830078D-11
Optimization completed.
-- Stationary point found.
Link
here
- BH3NH3
| File name | BH3NH3_OPRIMISATION_321G |
| File type | .log |
| Calculation type | FOPT |
| Calculation method | RB3LYP |
| Basis set | 3-21G |
| Charge | 0 |
| Spin | Singlet |
| E(RB3LYP) | -83.76661837 a.u. |
| RMS Gradient Norm | 0.00003006 a.u. |
| Imaginary Freq | |
| Dipole Moment | 5.84 Debye |
| Point Group | C1 |
| Job cpu time | 0 days 0 hours 0 minutes 29.0 seconds. |
Item Value Threshold Converged?
Maximum Force 0.000094 0.000450 YES
RMS Force 0.000030 0.000300 YES
Maximum Displacement 0.000419 0.001800 YES
RMS Displacement 0.000178 0.001200 YES
Predicted change in Energy=-5.742847D-08
Optimization completed.
-- Stationary point found.
| File name | BH3NH3_OPRIMISATION_631G |
| File type | .log |
| Calculation type | FOPT |
| Calculation method | RB3LYP |
| Basis set | 6-31G(d,p) |
| Charge | 0 |
| Spin | Singlet |
| E(RB3LYP) | -83.22468863 a.u. |
| RMS Gradient Norm | 0.00006021 a.u. |
| Imaginary Freq | |
| Dipole Moment | 5.56 Debye |
| Point Group | C1 |
| Job cpu time | 0 days 0 hours 0 minutes 33.0 seconds. |
Item Value Threshold Converged?
Maximum Force 0.000140 0.000450 YES
RMS Force 0.000039 0.000300 YES
Maximum Displacement 0.001015 0.001800 YES
RMS Displacement 0.000287 0.001200 YES
Predicted change in Energy=-1.238338D-07
Optimization completed.
-- Stationary point found.
Links
here here
Links
The optimisation file is liked to here
Frequency analysis
- NH3
Low frequencies --- -11.6313 -11.5960 -0.0028 0.0243 0.1402 25.5608 Low frequencies --- 1089.6620 1694.1733 1694.1736
Item Value Threshold Converged?
Maximum Force 0.000005 0.000450 YES
RMS Force 0.000003 0.000300 YES
Maximum Displacement 0.000011 0.001800 YES
RMS Displacement 0.000006 0.001200 YES
Predicted change in Energy=-8.408692D-11
Optimization completed.
-- Stationary point found.
Link
here
- BH3NH3
Low frequencies --- -29.4996 -0.0014 -0.0012 -0.0011 10.9904 16.6752 Low frequencies --- 261.5847 631.2506 637.7896
Item Value Threshold Converged?
Maximum Force 0.000273 0.000450 YES
RMS Force 0.000060 0.000300 YES
Maximum Displacement 0.001497 0.001800 YES
RMS Displacement 0.000435 0.001200 YES
Predicted change in Energy=-2.262680D-07
Optimization completed.
-- Stationary point found.
here
Reaction energies
- E(NH3)=-56.55776863 a.u.
- E(BH3)=-26.61532363 a.u.
- E(NH3BH3)= -83.22468863 a.u.
ΔE=E(NH3BH3)-[E(NH3)+E(BH3)]= -0.05159637 a.u =-135 kJ/mol
WEEK 2
Aromaticity
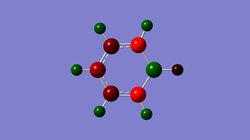
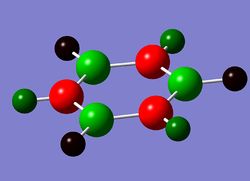
This week we are investigating the aromaticity and its relation to MO , as well as what happens when heteroatoms are introduced into the ring , while keeping the molecules isoelectronic. Comparision is going to be made between those analogues: boratabenzene, pyridinium and borazine.
Optimisation of benzene
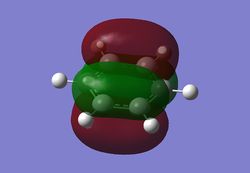
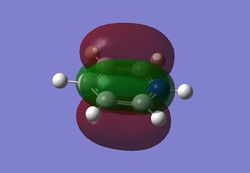
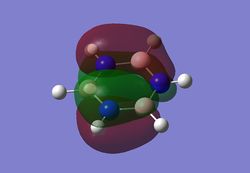
MO diagram
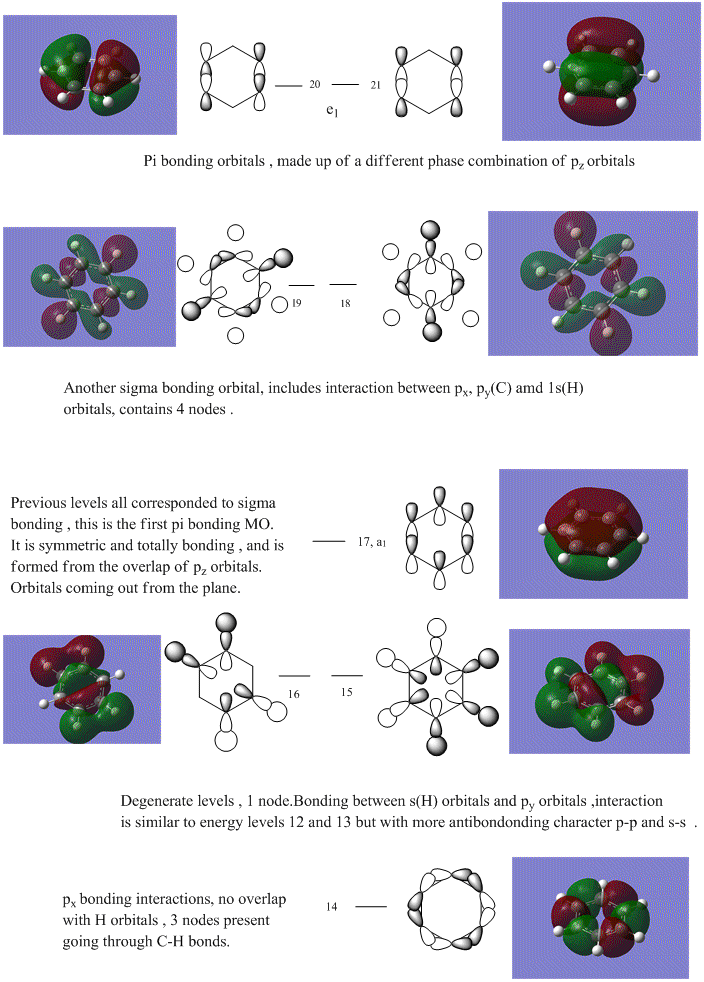
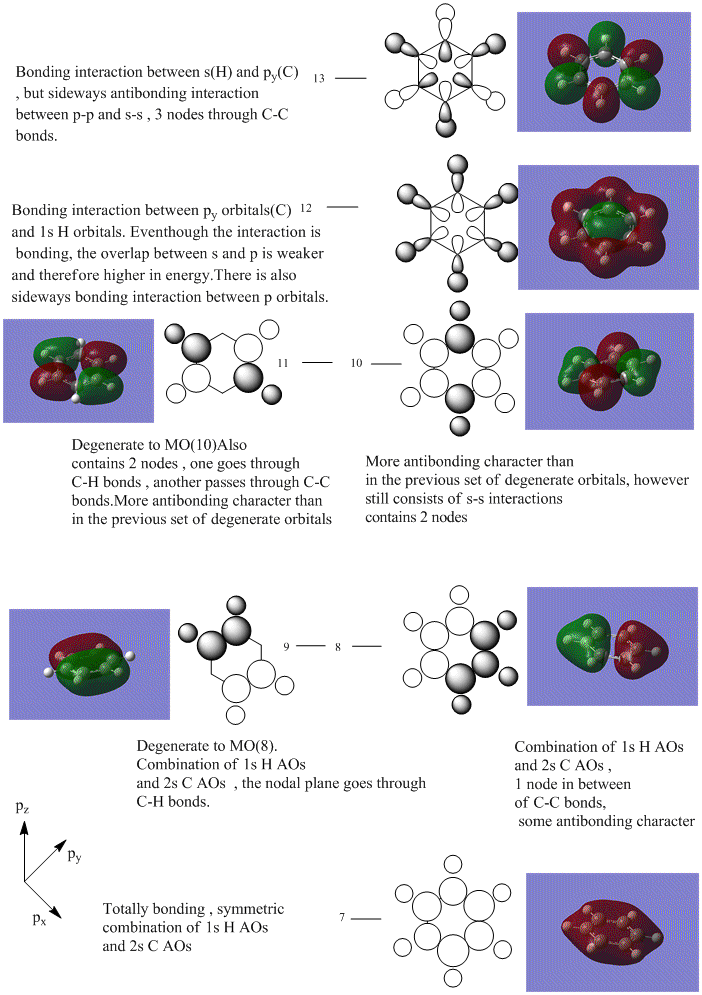
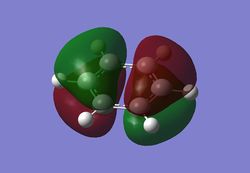
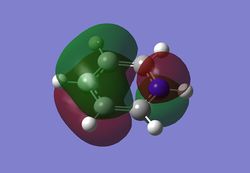
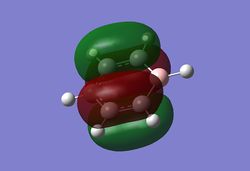
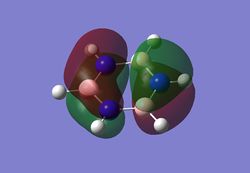
As can be seen from the MO diagram , there is a higher sigma bonding contribution than what is usually represented for benzene. High delocalisation of MOs over the whole structure of benzene aids aromaticity property. However classically it is taught most of the aromaticity arises from the electrons in 17,20,21 energy levels , as those electrons are involved in pi bonding which is weaker than sigma bonding and allows a higher degree of freedom . i.e they delocalise around the system better. For aromatic system 4n+2 are needed, each pz holds 1 electron , which gives 6 electrons in total.However as we can see there are more to bonding than just pi bonding , and electrons have a high probability of being in any of the MOs and therefore the system is much more delocalised as well as stabilised than it might have appeared to be.
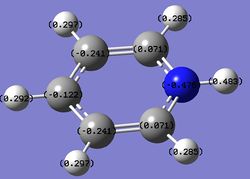
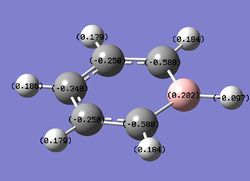
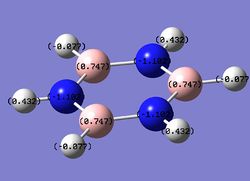
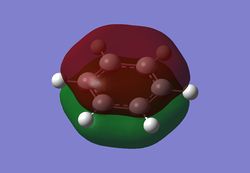
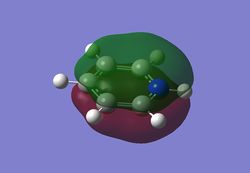
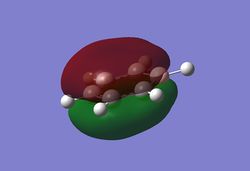
Charge distribution analysis
MO analysis
Summary
- Benzene Optimisation
benzene optimisation File Name benzene_optimisation_321G File Type .log Calculation Type FOPT Calculation Method RB3LYP Basis Set 3-21G Charge 0 Spin Singlet E(RB3LYP) -230.97574974 a.u. RMS Gradient Norm 0.00011813 a.u. Imaginary Freq Dipole Moment 0.00 Debye Point Group C1 Job cpu time: 0 days 0 hours 0 minutes 40.0 seconds.
Item Value Threshold Converged?
Maximum Force 0.000218 0.000450 YES
RMS Force 0.000080 0.000300 YES
Maximum Displacement 0.001064 0.001800 YES
RMS Displacement 0.000293 0.001200 YES
Predicted change in Energy=-5.022124D-07
Optimization completed.
-- Stationary point found.
benzene optimisation File Name benzene_optimisation_631G File Type .log Calculation Type FOPT Calculation Method RB3LYP Basis Set 6-31G(d,p) Charge 0 Spin Singlet E(RB3LYP) -232.25819628 a.u. RMS Gradient Norm 0.00004042 a.u. Imaginary Freq Dipole Moment 0.00 Debye Point Group C1 Job cpu time: 0 days 0 hours 2 minutes 4.8 seconds.
Item Value Threshold Converged?
Maximum Force 0.000090 0.000450 YES
RMS Force 0.000024 0.000300 YES
Maximum Displacement 0.000337 0.001800 YES
RMS Displacement 0.000103 0.001200 YES
Predicted change in Energy=-4.743663D-08
Optimization completed.
-- Stationary point found.
- Benzene frequency
Low frequencies --- -16.8786 -16.7630 -4.0197 -0.0009 -0.0006 0.0003
Low frequencies --- 414.2728 414.5526 620.8179
Item Value Threshold Converged?
Maximum Force 0.000135 0.000450 YES
RMS Force 0.000040 0.000300 YES
Maximum Displacement 0.000431 0.001800 YES
RMS Displacement 0.000160 0.001200 YES
Predicted change in Energy=-5.754679D-08
Optimization completed.
-- Stationary point found
- Boratabenzene optimisation
boratabenzene optimisation
File Name boratabenzene_optimisation_321G
File Type .log
Calculation Type FOPT
Calculation Method RB3LYP
Basis Set 3-21G
Charge -1
Spin Singlet
E(RB3LYP) -217.81415469 a.u.
RMS Gradient Norm 0.00004253 a.u.
Imaginary Freq
Dipole Moment 3.01 Debye
Point Group C1
Job cpu time: 0 days 0 hours 1 minutes 34.7 seconds.
Item Value Threshold Converged?
Maximum Force 0.000075 0.000450 YES
RMS Force 0.000021 0.000300 YES
Maximum Displacement 0.000274 0.001800 YES
RMS Displacement 0.000095 0.001200 YES
Predicted change in Energy=-4.529088D-08
Optimization completed.
-- Stationary point found.
boratabenzene optimisation
File Name boratabenzene_optimisation_631G
File Type .log
Calculation Type FOPT
Calculation Method RB3LYP
Basis Set 6-31G(d,p)
Charge -1
Spin Singlet
E(RB3LYP) -219.02053052 a.u.
RMS Gradient Norm 0.00003615 a.u.
Imaginary Freq
Dipole Moment 2.85 Debye
Point Group C1
Job cpu time: 0 days 0 hours 2 minutes 48.2 seconds.
Item Value Threshold Converged?
Maximum Force 0.000061 0.000450 YES
RMS Force 0.000018 0.000300 YES
Maximum Displacement 0.000279 0.001800 YES
RMS Displacement 0.000090 0.001200 YES
Predicted change in Energy=-3.774403D-08
Optimization completed.
-- Stationary point found.
- Boratabenzene frequency
Low frequencies --- -12.4137 -0.0007 0.0007 0.0010 13.9750 18.0393 Low frequencies --- 371.3802 404.1483 565.1934
Item Value Threshold Converged?
Maximum Force 0.000118 0.000450 YES
RMS Force 0.000036 0.000300 YES
Maximum Displacement 0.000319 0.001800 YES
RMS Displacement 0.000130 0.001200 YES
Predicted change in Energy=-4.280655D-08
Optimization completed.
-- Stationary point found.
- Pyridinium optimisation
pyridinium optimisation
File Name pyridinium_optimisation_321G
File Type .log
Calculation Type FOPT
Calculation Method RB3LYP
Basis Set 3-21G
Charge 1
Spin Singlet
E(RB3LYP) -247.29364861 a.u.
RMS Gradient Norm 0.00003627 a.u.
Imaginary Freq
Dipole Moment 1.95 Debye
Point Group C1
Job cpu time: 0 days 0 hours 1 minutes 15.1 seconds.
Item Value Threshold Converged?
Maximum Force 0.000074 0.000450 YES
RMS Force 0.000022 0.000300 YES
Maximum Displacement 0.000479 0.001800 YES
RMS Displacement 0.000132 0.001200 YES
Predicted change in Energy=-4.802923D-08
Optimization completed.
-- Stationary point found.
pyridinium optimisation
File Name pyridinium_optimisation_631G
File Type .log
Calculation Type FOPT
Calculation Method RB3LYP
Basis Set 6-31G(d,p)
Charge 1
Spin Singlet
E(RB3LYP) -248.66807392 a.u.
RMS Gradient Norm 0.00004768 a.u.
Imaginary Freq
Dipole Moment 1.87 Debye
Point Group C1
Job cpu time: 0 days 0 hours 2 minutes 57.1 seconds.
Item Value Threshold Converged?
Maximum Force 0.000086 0.000450 YES
RMS Force 0.000028 0.000300 YES
Maximum Displacement 0.000678 0.001800 YES
RMS Displacement 0.000207 0.001200 YES
Predicted change in Energy=-1.044727D-07
Optimization completed.
-- Stationary point found.
- Pyridinium frequency
Low frequencies --- -6.9820 -0.0010 -0.0005 0.0001 17.3242 18.4312 Low frequencies --- 392.5033 404.0208 620.5149
Item Value Threshold Converged?
Maximum Force 0.000153 0.000450 YES
RMS Force 0.000048 0.000300 YES
Maximum Displacement 0.000756 0.001800 YES
RMS Displacement 0.000277 0.001200 YES
Predicted change in Energy=-1.086647D-07
Optimization completed.
-- Stationary point found.
- Borazine Optimisation
Borazine optimisation
File Name borazine_optimisation_321G
File Type .log
Calculation Type FOPT
Calculation Method RB3LYP
Basis Set 3-21G
Charge 0
Spin Singlet
E(RB3LYP) -241.35697019 a.u.
RMS Gradient Norm 0.00006111 a.u.
Imaginary Freq
Dipole Moment 0.00 Debye
Point Group C1
Job cpu time: 0 days 0 hours 1 minutes 35.1 seconds.
Item Value Threshold Converged?
Maximum Force 0.000098 0.000450 YES
RMS Force 0.000032 0.000300 YES
Maximum Displacement 0.000323 0.001800 YES
RMS Displacement 0.000098 0.001200 YES
Predicted change in Energy=-9.089172D-08
Optimization completed.
-- Stationary point found.
benzene optimisation
File Name benzene_optimisation_631G
File Type .log
Calculation Type FOPT
Calculation Method RB3LYP
Basis Set 6-31G(d,p)
Charge 0
Spin Singlet
E(RB3LYP) -232.25819628 a.u.
RMS Gradient Norm 0.00004042 a.u.
Imaginary Freq
Dipole Moment 0.00 Debye
Point Group C1
Job cpu time: 0 days 0 hours 2 minutes 4.8 seconds.
Item Value Threshold Converged?
Maximum Force 0.000090 0.000450 YES
RMS Force 0.000024 0.000300 YES
Maximum Displacement 0.000337 0.001800 YES
RMS Displacement 0.000103 0.001200 YES
Predicted change in Energy=-4.743663D-08
Optimization completed.
-- Stationary point found.
- Borazine frequency
Low frequencies --- -16.8786 -16.7630 -4.0197 -0.0009 -0.0006 0.0003 Low frequencies --- 414.2728 414.5526 620.8179
Item Value Threshold Converged?
Maximum Force 0.000135 0.000450 YES
RMS Force 0.000040 0.000300 YES
Maximum Displacement 0.000431 0.001800 YES
RMS Displacement 0.000160 0.001200 YES
Predicted change in Energy=-5.754679D-08
Optimization completed.
-- Stationary point found
Links
- Benzene
Optimisation
DOI:10042/22013 DOI:10042/22033
Frequency
DOI:10042/22035
NBO
DOI:10042/22036
- Boratabenzene
Optimisation
DOI:10042/22038 DOI:10042/22037
Frequency
DOI:10042/22039
NBO
DOI:10042/22040
- Pyridinium
Optimisation
DOI:10042/22041 DOI:10042/22042
Frequency
DOI:10042/22043
NBO
DOI:10042/22044
- Borazine
Optimsation
DOI:10042/22045 DOI:10042/22046
Frequency
DOI:10042/22047
NBO
DOI:10042/22048