Rep:Mod:XYZ1234
The Hydrogenation of the Cyclopentadiene Dimer
Kinetic or thermodynamic control for the cyclodimerisation of cyclopetadiene?
A cyclodimerisation occurs between two cyclopentadiene leading to the two following molecules:
The endo dimer is specifically produced. Our aim is then to know if the reaction undergoes a thermodynamic or kinetic control. To achieve that we can perform calculations with Chemdraw3D, which will provide different informations about the stretching, the bending, the torsion, van der Waals and hydrogen bonding of the two molecules. The results will be collected in a table and treated later.
The endo dimer can be hydrogenated leading to two new molecules which are themselves able to undergo a further hydrogenation (after a long time). We are now interested to discover which one of the two new dimer (3 and 4)is the most stable in a thermodynamic sense.
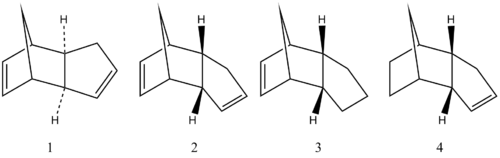
In the table below are collected the different informations regarding the four molecules.
| Property | Dimer 1 | Dimer 2 | Dimer 3 | Dimer 4 |
|---|---|---|---|---|
| Stretch | 1.2850 | 1.2510 | 1.2783 | 1.0961 |
| Bend | 20.5795 | 20.8480 | 19.8622 | 14.5218 |
| Stretch-bend | -0.8377 | -0.8361 | -0.8349 | -0.5492 |
| Torsion | 7.6561 | 9.5112 | 10.8066 | 12.4983 |
| 1,4 VDW | 4.2332 | 4.3213 | 5.6330 | 4.5130 |
| Dipole/Dipole | 0.3775 | 0.4478 | 0.1621 | 0.1406 |
| Total energy | 31.8765 kcal/mol | 33.9976 kcal/mol | 35.6851 kcal/mol | 31.1520 kcal/mol |
Stereochemistry and reactivity of an Intermediate in the Synthesis of Taxol
In one of the steps of the synthesis of Taxol, a drug used for the treatment of different cancers (mainly lung, ovarian et breast cancers)a bridged intermediate is produced. This step leads to two atropisomers (they only differ by the rotation of a single bond). Of course those two intermediates possess different stabilities and different reactivities as well. We will now investigate what the main differences in stabilities as well in conformations are.
ah_test |
Ah_taxol_mol_2 |
| Property | MM2 (1) | MMFF94 (1) | MM2 (2) | MMFF94 (2) |
|---|---|---|---|---|
| Stretch | 2.6210 | 2.5492 | ||
| Bend | 11.3420 | 11.3736 | ||
| Stretch-bend | 0.3432 | 0.3204 | ||
| Torsion | 19.6668 | 17.3691 | ||
| 1,4 VDW | 12.8708 | 12.7401 | ||
| Dipole/Dipole | -2.0022 | -1.6995 | ||
| Total energy | 42.6829 kcal/mol | 60.5651 kcal/mol | 40.3902 kcal/mol | 60.6848 kcal/mol |
As we can see in the table above we get different results with the two methods. Indeed with the MM2 method the atropisomer 2 is more stable whereas with the MMFF94 method it is the contrary. But the difference is really slight with the second method and probably unrelevant.
Regarding the alkene we can explain its low reactivity with two main arguments. Firstly the electron density is really poor around the bond and then does not undergo electrophilic addition. On the pther hand the overlap of the pi-bond is not good as shown by the measurement of the dihedral angles.
Regioselective addition of dichlorocarbene
Monosaccharide chemistry: glycosidation