Rep:Mod:XYZ0842
NH3
Optimising NH3
Calculation Method = RB3LYP
Basis Set= 6-31G(d,p)
Final Energy E(RB3LYP) = -56.55776873 a.u
RMS Gradient = 0.00000485 a.u
Point Group = C3V
Optimised N-H bond distance = 1.018Å
Optimised H-N-H bond angle = 105.741°
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000072 0.001800 YES
RMS Displacement 0.000035 0.001200 YES
Ammonia |
The optimisation file is linked here: [[1]]
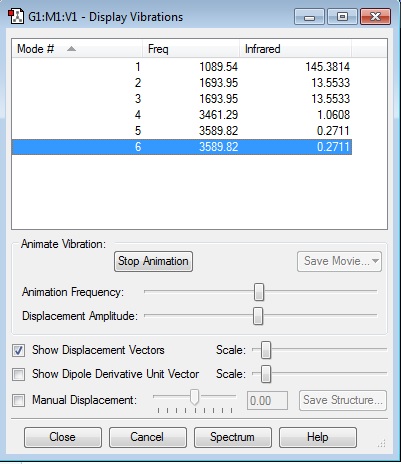
Vibrations and Charges
Vibrational Modes of NH3
From the 3N-6 rule, it is expected that there will be 6 modes of vibration as N=4; there are 4 atoms in the molecule.
Modes 2 and 3, with frequency 1693.95 Hz are degenerate. Modes 5 and 6, with frequency 3589.82 Hz are degenerate.
"Bending Vibrations" are modes 1,2 and 3. "Bond Stretch Vibrations" are modes 4,5 and 6.
Mode 4 is highly symmetric.
Mode 1 is the "Umbrella mode".
There should be 4 bands displayed in an experimental spectrum of NH3 gas.
NH3 Charges
N charge = -1.125
H charge (for all 3 atoms) = 0.375
These charges are calculated using GaussView. As nitrogen is more electronegative that hydrogen, it is expected to have a negative charge as it attracts the charge density more than hydrogen, hence hydrogen is positively charged.
N2
Optimising N2
Calculation Method = RB3LYP
Basis Set= 6-31G(d,p)
Final Energy E(RB3LYP) = -109.52412868 a.u
RMS Gradient = 0.00000060 a.u
Point Group = D∞h
Optimised N≡N bond distance = 1.106Å
Optimised N≡N bond angle = 180.0°
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES
Nitrogen |
The singular frequency associated with this molecule = 2457.33Hz
As a nitrogen molecule is diatomic, the atoms are identical and thus the charge is evenly distributed over both atoms, with no dipole moment present.
The optimisation file is linked here: [[2]]
H2
Optimising H2
Calculation Method = RB3LYP
Basis Set= 6-31G(d,p)
Final Energy E(RB3LYP) = -1.17853936 a.u
RMS Gradient = 0.00000017 a.u
Point Group = D∞h
Optimised H-H bond distance = 0.743Å
Optimised H-H bond angle = 180°
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
Hydrogen |
The singular frequency associated with this molecule = 4465.68Hz
As a hydrogen molecule is diatomic, the atoms are identical and thus the charge is evenly distributed over both atoms, with no dipole moment present.
The optimisation file is linked here: [[3]]
Reactions and Orbitals
Haber-Bosch Reaction Energy Calculation
E(NH3)= -56.55776873 a.u
2E(NH3)= -113.11553746 a.u
E(N2)= -109.52412868 a.u
E(H2)= -1.17853936 a.u
3E(H2)= -3.53561808 a.u
ΔE=2E(NH3)-[E(N2)+3E(H2)] = -0.05790700 a.u = -146.49 kJ/mol
-149.49 kJ/mol is the energy required to produce ammonia gas from hydrogen gas and nitrogen gas. The ammonia product is more stable than the reactants.
Cl2
Optimising Cl2
Calculation Method = RB3LYP
Basis Set= 6-31G(d,p)
Final Energy E(RB3LYP) = -920.34987886 a.u
RMS Gradient = 0.00002510 a.u.
Point Group = D∞h
Optimised H-H bond distance = 2.042Å
Optimised H-H bond angle = 180°
Item Value Threshold Converged? Maximum Force 0.000043 0.000450 YES RMS Force 0.000043 0.000300 YES Maximum Displacement 0.000121 0.001800 YES RMS Displacement 0.000172 0.001200 YES
Chlorine |
Cl2 is a linear, diatomic and uncharged molecule. As such, it is predicted that it has 3N-5 modes of vibration. N=2, so chlorine should have a singular mode of vibration and thus display one frequency after optimisation.
The calculated bond stretching vibration for chlorine is at 520.32 Hz.
Cl2 is uncharged according to the calculations; both atoms have a relative charge of 0.000 as they are identical and thus the charge is evenly distributed of the molecule. There is no dipole moment present in the molecule.
The optimisation file is linked here: [[4]]
Molecular Orbitals - Cl2
Cl2 has the electronic configuration: 1s22s22p63s2
HCl
Optimising HCl
Calculation Method = RB3LYP
Basis Set= 6-31G(d,p)
Final Energy E(RB3LYP) = -460.80077875 a.u
RMS Gradient = 0.00005211 a.u.
Point Group = C∞V
Optimised H-H bond distance = 1.286Å
Optimised H-H bond angle = 180°
Item Value Threshold Converged? Maximum Force 0.000090 0.000450 YES RMS Force 0.000090 0.000300 YES Maximum Displacement 0.000139 0.001800 YES RMS Displacement 0.000197 0.001200 YES
HCl |
As HCl is linear, there is only one associated frequency at 2956.80 Hz as predicted by the 3N-5 rule. N=2, hence there is only one mode of vibration: bond stretching. The Cl is more electronegative and has a charge of -0.284. The molecule is neutral and the hydrogen atom has a positive charge of 0.284.
The optimisation file is linked here: [[5]]