Rep:Mod:Wyd123
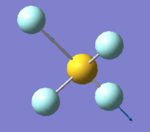
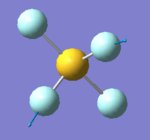
NH3
NH3 |
Optimisation of NH3
| Molecule | NH3 |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(D,P) |
| Final Energy E(RB3LYP) | -56.55776873 a.u. |
| RMS gradient | 0.00000485 a.u. |
| Point Group | C3V |
| Optimised N-H Bond Distance | (1.02 ± 0.01)Å |
| Optimised H-N-H Bond Angle | (106 ± 1)° |
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000072 0.001800 YES
RMS Displacement 0.000035 0.001200 YES
The optimisation file is linked to here
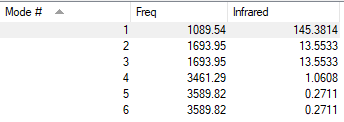
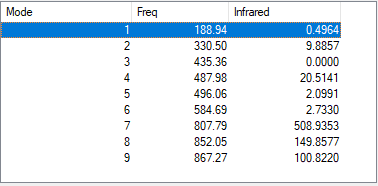
Vibrations Analysis of NH3
| Wavenumber | Symmetry | Intensity (arbitrary units) | Image | |
| 1090 | A1 | 145 | 
| |
| 1694 | E | 14 | 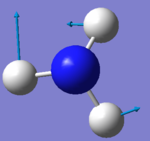
| |
| 1694 | E | 14 | 
| |
| 3461 | A1 | 1 | 
| |
| 3590 | E | 0 | 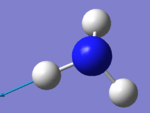
| |
| 3590 | E | 0 | 
|
Q1) How many modes do you expect from the 3N-6 rule?
A1) Number of modes=3(4)-6=6 modes
Q2) Which modes are degenerate (ie have the same energy)?
A2) The two modes at 1694 cm-1 and the two modes at 3590 cm-1.
Q3) Which modes are "bending" vibrations and which are "bond stretch" vibrations?
A3) Bending Vibrations are 1090cm-1 and 1694cm-1. Stretching Vibrations are 3461cm-1 and 3590cm-1.
Q3) which mode is highly symmetric?
A3) The mode at 3461cm-1.
Q4) One mode is known as the "umbrella" mode, which one is this?
A4) The mode at 1090cm-1.
Q5) How many bands would you expect to see in an experimental spectrum of gaseous ammonia?
A5) I would expect to see 2 bands, one at 145 a.u. and one at 14 a.u. The one at 1 a.u. is too low in intensity to see.
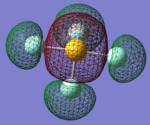
Charge Distributions in NH3
Charge on N atom: -1.125
Charge on H atoms: +0.375
Before using Gaussian, I already expected the N atom to hold the negative charge, while the two H atoms to hold the positive charge. This is because N is much more electronegative than H, hence will pull the electron density away from the two H atoms.
N2
N2 |
Optimisation of N2
| Molecule | N2 |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(D,P) |
| Final Energy E(RB3LYP) | -109.52412868 a.u. |
| RMS gradient | 0.00000060 a.u. |
| Point Group | D∞h |
| Optimised N-N Bond Distance | (1.11 ± 0.01)Å |
Item Value Threshold Converged?
Maximum Force 0.000001 0.000450 YES
RMS Force 0.000001 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000000 0.001200 YES
The optimisation file is linked to here
Comparison of N2 Bond Distance in Coordinated Structures
The reported N2 Bond Distance in Crystal Structure of YECMOL is: 1.108Å The link to the structure is: https://www.ccdc.cam.ac.uk/structures/Search?Ccdcid=YECMOL&DatabaseToSearch=Published
The N2 triple bond distance in the Ruthenium Tansition Metal Complex is longer than the optimised values of 1.10550Å. A ligand bond is formed when the non-bonding d orbitals of the Ru metal overlap with the π* anti-orbitals of N. The electron in the non-bonding d orbitals of the transition metal will drift into the π* anti-orbitals orbitals of the dinitrogen ligand, destabilizing the N2 triple bond, causing it to become weaker, hence slightly longer. This will also result in structural change in the Metal complex. However, it does not necessarily increase the strength of the bond between Ru and N. [1]
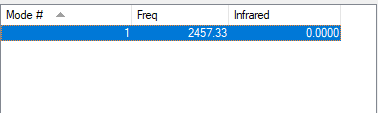
Vibrations Analysis of N2
| Wavenumber | Symmetry | Intensity (arbitrary units) | Image | |
| 2457 | SGG | 0.0000 |
Charge Distributions in N2
Charge on N atoms: 0.000
It is a linear diatomic molecule.
H2
H2 |
Optimisation of H2
| Molecule | H2 |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(D,P) |
| Final Energy E(RB3LYP) | -1.17853936 a.u. |
| RMS gradient | 0.00000017 a.u. |
| Point Group | D∞h |
| Optimised H-H Bond Distance | (0.74 ± 0.01)Å |
Item Value Threshold Converged?
Maximum Force 0.000000 0.000450 YES
RMS Force 0.000000 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000001 0.001200 YES
The optimisation file is linked to here
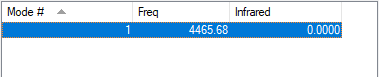
Vibrations Analysis of H2
| Wavenumber | Symmetry | Intensity (arbitrary units) | Image | |
| 4466 | SGG | 0.0000 | 
|
Charge Distributions in H2
Charge on H atoms: 0.000
It is a linear diatomic molecule.
Calculations for The Haber-Bosch process
The Haber-Bosch Process is: N2 + 3H2 -> 2NH3
E(NH3)= -56.55776873 a.u.
2*E(NH3)= -113.11553746 a.u.
E(N2)= -109.52412868 a.u.
E(H2)= -1.17853936 a.u.
3*E(H2)= -3.53561808 a.u.
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -0.0557907 a.u.= -146.5 kJ mol-1
The ammonia product is more stable than the reactants as it is deeper in energy. Also, based on the calculations of the change in energy, the reaction is exothermic. Hence, forming the ammonia product is thermodynamically more favorable as it is more favorable for reactions to occur where products tend to a lower energy state.
CO
CO |
Optimisation of CO
| Molecule | CO |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(D,P) |
| Final Energy E(RB3LYP) | -113.30945314 a.u. |
| RMS gradient | 0.00000693 a.u. |
| Point Group | C∞v |
| Optimised C≡O Bond Distance | (1.14 ± 0.01)Å |
Item Value Threshold Converged?
Maximum Force 0.000012 0.000450 YES
RMS Force 0.000012 0.000300 YES
Maximum Displacement 0.000005 0.001800 YES
RMS Displacement 0.000007 0.001200 YES
The optimisation file is linked to here
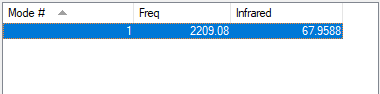
Vibrations Analysis of CO
| Wavenumber | Symmetry | Intensity (arbitrary units) | Image | Type of Vibration |
| 2209 | SG | 68 |  |
Symmetrical Stretch |
I would expect to see 1 band at 68 a.u.
Charge Distributions in CO
Charge on C atom: +0.506
Charge on O atoms: -0.506
The O atom is more electronegative than the C atom, hence carries the negative charge. However, the overal charge of the CO is 0.
Molecular Orbitals of CO
SF4
SF4 |
Optimisation of SF4
| Molecule | SF4 |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(D,P) |
| Final Energy E(RB3LYP) | -797.45952464 a.u. |
| RMS gradient | 0.00005908 a.u. |
| Point Group | C2v |
| Optimised S-F Bond Distance | (1.59 ± 0.01)Å, (1.67 ± 0.01)Å |
| Optimised F-S-F Bond Angle | (87 ± 1)°, (171 ± 1)°, (102 ± 1)° |
There are three different F-S-F Bond Angles due to the fact that in order to reach a thermodynamically stable energetic level, the F atoms and the central S atom arrange themselves in a way to minimize the electron repulsion due to the two lone pairs on S.
Item Value Threshold Converged?
Maximum Force 0.000108 0.000450 YES
RMS Force 0.000051 0.000300 YES
Maximum Displacement 0.000437 0.001800 YES
RMS Displacement 0.000270 0.001200 YES
The optimisation file is linked to here
Vibrations Analysis of SF4
Charge Distributions in SF4
Charge on S atom: +1.984
Charge on F atoms: -0.455, -0.537
The charge are different for the F atoms. Two F atoms closer to the two lone pairs of electrons on S are -0.455 while the two F atoms further away from the two lone pair of electrons on S are -0.537. This is to minimize electron repulsion.
Molecular Orbitals of SF4
References
- ↑ J. Chatt. Molecular Nitrogen as a Ligand, 1970, 24, 425-442..
Marking
Note: All grades and comments are provisional and subject to change until your grades are officially returned via blackboard. Please do not contact anyone about anything to do with the marking of this lab until you have received your grade from blackboard.
Wiki structure and presentation 1/1
Is your wiki page clear and easy to follow, with consistent formatting?
YES
Do you effectively use tables, figures and subheadings to communicate your work?
YES
NH3 1/1
Have you completed the calculation and given a link to the file?
YES
Have you included summary and item tables in your wiki?
YES
Have you included a 3d jmol file or an image of the finished structure?
YES
Have you included the bond lengths and angles asked for?
YES
Have you included the “display vibrations” table?
YES
Have you added a table to your wiki listing the wavenumber and intensity of each vibration?
YES
Did you do the optional extra of adding images of the vibrations?
YES
Have you included answers to the questions about vibrations and charges in the lab script?
YES - for future work please state wavenumbers when discussing vibrational spectra and not the intensities of the bands (see A5). your discussion is still valid!
N2 and H2 0.5/0.5
Have you completed the calculations and included all relevant information? (summary, item table, structural information, jmol image, vibrations and charges)
YES- you could have explained that electronegativities are the same and therefore no charges are expected on homonuclear diatomics.
Crystal structure comparison 0.5/0.5
Have you included a link to a structure from the CCDC that includes a coordinated N2 or H2 molecule?
YES
Have you compared your optimised bond distance to the crystal structure bond distance?
YES - You tried to give a reference for your discussion on N2 as a Ligand in TM complexes, this is good! You missed to include the abbreviation of the corresponding journal.
Haber-Bosch reaction energy calculation 1/1
Have you correctly calculated the energies asked for? ΔE=2*E(NH3)-[E(N2)+3*E(H2)]
YES
Have you reported your answers to the correct number of decimal places?
YES
Do your energies have the correct +/- sign?
YES
Have you answered the question, Identify which is more stable the gaseous reactants or the ammonia product?
YES
Your choice of small molecule 4/5
Have you completed the calculation and included all relevant information?
YES - you could have calculated the number of expected vibrational modes for CO and explain why you expect to see one band in its spectrum but for other diatomics (H2 and N2) you are not.
Have you added information about MOs and charges on atoms?
YES
You correctly identified contributing AOs, occupancy, relative energies and the effect on bonding. You could have been more specific when labelling contributing AOs, e.g such as stating 2pz orbitals to be the contributing ones in the 4th displayed MO rather than just stating 2p orbitals. You could have commented on the relative energies of the MOs (e.g. number of nodes). The 4th and 5th displayed MOs are not only in the HOMO LUMO region, they are representing the HOMO and the LUMO of CO respectively.
Independence 1/1
If you have finished everything else and have spare time in the lab you could: Check one of your results against the literature, or Do an extra calculation on another small molecule, or
YES - you could have analysed the calculated vibrations in more depth.
Do some deeper analysis on your results so far