Rep:Mod:Wlt113
Introduction
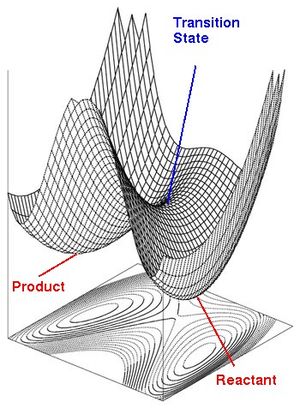
Much of chemical kinetics today is fundamentally based upon transition state theory (TST), which postulates that the transition state is a theoretical high-energy state that reacting molecules can attain.[1] Mathematically, it is a type of stationary point, and as shown in Figure 1, can appear as either a maximum point in a 1-dimensional (1D) plot, or a saddle point in a 2-dimensional potential energy surface (PES) plot.[1] The saddle point in the 2D PES divides the stable potential wells of the reactants and products, and represents a dividing surface where there is only one direction to proceed in without elevating the system's energy. In addition, the reaction coordinate taken in the 1D plot often represents the minimum energy path (MEP) in the 2D PES leading from the reactants to the products.[1]
| (a) | (b) |
|---|---|
 |
 |
Using computational methods, it is possible to calculate and compare the energies of the reactants, transition state and products, and assess the reactivity of various pathways for a reaction by comparing activation energies and reaction free energy changes or reaction energies. In this work, using the computational software Gaussian at the semi-empirical PM6 and hybrid functional density functional theory (DFT) method B3LYp/6-31G(d),[3] the transition states of several Diels-Alder reactions are investigated to shed insight on the kinetics and thermodynamics of the pathways possible, taking into account stereoelectronic effects where relevant.
Nf710 (talk) 22:26, 8 March 2017 (UTC) You have shown a fairly good understanding here. However you should have talked about the TS and minima in terms of second derivatives.
Exercise 1: Reaction of Butadiene with Ethene
Reaction Scheme
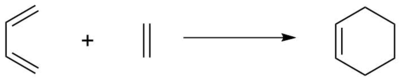
In this exercise, the Diels-Alder cycloaddition between butadiene and ethene was studied, and the transition state optimized using Gaussian at the PM6 level. Links to the calculation output for the optimized structures of butadiene,[4] ethene,[5] cyclohexene,[6] the transition state,[7] and the intrinsic reaction coordinate (IRC)[8] can be found in References.
Orbital Symmetry-controlled Overlap of Frontier MOs of Butadiene and Ethene
The reactivity of the reactants in a Diels-Alder cycloaddition can be rationalized using frontier molecular orbital (FMO) theory.[9] As shown in Figure 2, the highest occupied molecular orbitals (HOMOs) and lowest unoccupied molecular orbitals (LUMOs) of ethene and butadiene can overlap to form key FMOs in the transition state and resultant cyclohexene product. An MO can be symmetric (having a plane of symmetry) or antisymmetric (lacking a plane of symmetry), and as shown in the MO diagram, the symmetry requirements in such a reaction are that reactant MOs of the same symmetry must interact for the orbital overlap to be non-zero. The overlap integrals for the three possible cases where reactant MOs can be either symmetric or antisymmetric are shown below (Table 2). These results are consistent with the Woodward-Hoffmann rules for pericyclic reactions (Figure 3), which predicts that the reaction is thermally allowed whether the HOMO of butadiene overlaps with the LUMO of ethene (normal electron-demand Diels-Alder reaction) or the HOMO of ethene overlaps with the LUMO of butadiene (inverse electron-demand).[10] Since there is significant overlap in both cases, this system may be considered an example of a neutral Diels-Alder reaction [11]

(Fv611 (talk) 13:15, 6 March 2017 (UTC) Very good MO diagram.)
| MO | Butadiene | Ethene | Cyclohexene | ||
|---|---|---|---|---|---|
| LUMO+1 | N.A. | N.A. | |||
| LUMO |  |
 |
|||
| HOMO |  |
 |
|||
| HOMO-1 | N.A. | N.A. |
| Symmetries of Reactant MOs | Overlap Integral | Reaction allowed? |
|---|---|---|
| Symmetric-Symmetric | Non-zero | Allowed |
| Symmetric-Antisymmetric | Zero | Forbidden |
| Antisymmetric-Antisymmetric | Non-zero | Allowed |
(Fv611 (talk) 13:15, 6 March 2017 (UTC) Could have discussed this instead of just giving a table.)

Variation in C-C Bond Lengths with Reaction Coordinate
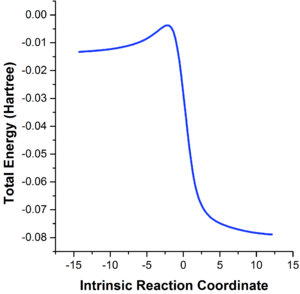
The lengths of the C-C bonds within reactant molecules vary continuously with reaction coordinate, as shown in Figure 4, which was obtained via an intrinsic reaction coordinate (IRC) calculation that is based on following the MEP from reactants to products. The computed C-C bond lengths within the reactants, transition state and product are tabulated in Table 3.
The C1-C6 and C4-C5 bonds (1.33 Å) are initially slightly shorter than the typical sp2-sp2 C=C double bond length (1.34 Å) in butadiene, due to π-conjugation, but subsequently surpasses 1.34 Å and increases to 1.37 Å in the transition state, ultimately becoming single bonds (1.50 Å) in the cyclohexene product with the typical sp2-sp3 C-C bond length (1.50 Å).[12] The C4-C5 bond is initially a typical sp2-sp2 C-C bond (1.47 Å), but decreases in length in the transition state (1.41 Å), until it reaches a typical sp2-sp2 C=C bond.[12]
The C2-C3 C=C bond in ethene (1.33 Å) is slightly shorter than the typical sp2-sp2 C=C bond length; as the reaction progresses, it lenghthens to being intermediate to typical sp2-sp2 and sp3-sp3 C-C bond lengths (1.38 Å), and eventually becomes a typical sp3-sp3 C-C bond.[12]
Within the transition state, the C1-C2 and C3-C4 bond length of 2.11 Å is intermediate between a typical sp3-sp3 C-C bond (1.54 Å)[12] and the sum of Van der Waals (VDW) radii for two carbon atoms (3.40 Å).[13] The distance between two atoms being under the sum of their VDW radii is the indication of a bonding interaction, which is the case of the two partially formed C1-C2/C3-C4 bonds in the transition state.
| Butadiene | Ethene | Transition State | Cyclohexene | |
|---|---|---|---|---|
| C1-C2 | N.A. | N.A. | 2.11 | 1.54 |
| C2-C3 | N.A. | 1.33 | 1.38 | 1.53 |
| C3-C4 | N.A. | N.A. | 2.11 | 1.54 |
| C4-C5 | 1.33 | N.A. | 1.38 | 1.50 |
| C5-C6 | 1.47 | N.A. | 1.41 | 1.34 |
| C1-C6 | 1.33 | N.A. | 1.38 | 1.50 |

Visualization of Reaction Coordinate
As shown in Figure 5, corresponding to the vibration with an imaginary frequency of 948.83i cm-1, formation of the two C-C bonds between butadiene and ethene in the transition state is a synchronous and concerted process, as expected for the symmetrical system where two equivalent C-C bonds are formed.
| Front View | Side View |
|---|---|
 |
 |
Exercise 2: Reaction of Cyclohexadiene and 1,3-Dioxole
Reaction Scheme
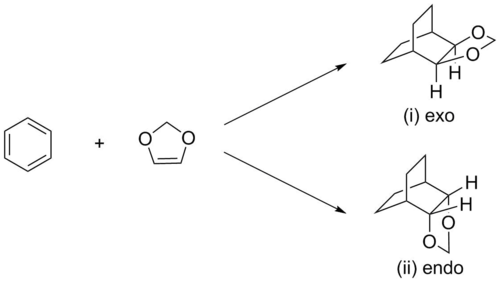
(You're missing the distinguishing double bonds in the products Tam10 (talk) 12:51, 8 March 2017 (UTC))
In this exercise, the Diels-Alder reaction between the electron-rich dienophile 1,3-dioxole and the diene cyclohexadiene was studied, and the transition state was initially optimized at PM6, before being reoptimized at the B3LYP/6-31G(d) level of theory. Links to the calculation output for the PM6 and B3LYP/6-31G(d)-optimized structures of cyclohexadiene,[14] [15] 1,3-dioxole,[16] [17] the exo product,[18] [19] the endo product,[20] [21] the exo transition state,[22] [23] the endo transition state,[24] [25] and the intrinsic reaction coordinate (IRC) of the exo pathway[26] [27] and endo pathway[28][29] can be found in References.
Overlap of FMOs of Cyclohexadiene and 1,3-Dioxole
The orbital symmetry-controlled overlap of reactant FMOs are shown in Figure 6. This is an example of an inverse electron-demand Diels-Alder reaction as the HOMO in the system is that of the 1,3-dioxole dienophile, whereas the LUMO is contributed by cyclohexadiene.
| Exo | Endo |
|---|---|
 |
 |
Kinetics and Thermodynamics of Reaction Pathways
The activation energies and reaction enthalpies for the formation of the exo and endo adducts respectively were calculated by taking differences in the values of Sum of electronic and thermal Free energy obtained for the reactants, transition state and products through Gaussian (Table 5). It was found that the endo product was both the kinetically (lower activation energy) and thermodynamically (larger, more negative reaction enthalpy) favoured product, as compared to the exo product.
| Computed Energy of Species (EH) | Activation Energy (kJ mol-1) | Reaction Energy (kJ mol-1) | ||
|---|---|---|---|---|
| Reactants | Cyclohexadiene | -233.324375 | N.A. | N.A. |
| 1,3-Dioxole | -267.068643 | |||
| Sum of Reactant Energies | -500.393018 | |||
| Transition State | Exo | -500.285411 | 282.52 | N.A. |
| Endo | -500.332149 | 159.81 | ||
| Product | Exo | -500.417322 | N.A. | -63.81 |
| Endo | -500.418692 | -67.41 | ||
Nf710 (talk) 22:34, 8 March 2017 (UTC) Your activation energies are incorrect for the exo you used the Sum of electronic and thermal Energies rather than Sum of electronic and thermal free Energies. This is a shame as your energy of reaction is correct for both.
Secondary Orbital Interactions and Steric Factors influencing Stereoselectivity in the Transition State
The lower activation energy for endo product formation can be explained by secondary orbital interactions. As shown in Table 6, the p-orbital coefficients on the oxygen atoms are of the correct phase to and can overlap with the orbital coefficients on the diene carbon atoms not directly involved in bond formation. In addition, in the exo transition state, the steric repulsion non-reacting atoms of the reactants (as illustrated in Table 6) is destabilizing. Hence, both electronic and steric factors favour the endo transition state, leading to the computed kinetic and thermodynamic stereoselectivity. The steric clash within the exo geometry also raises the energy of the exo product, leading to the endo product being the thermodynamically favoured one.
Nf710 (talk) 22:40, 8 March 2017 (UTC) Very good section. Nice diagrams explaining all your points. Shame you got the exo activation energy wrong.
Exercise 3: Comparing the Diels-Alder and Cheletropic Reactions between o-Xylylene and SO2
Reaction Scheme

In this final exercise, the expected exo and endo hetero-Diels-Alder reaction pathways, the alternative exo and endo Diels-Alder reaction, and the cheletropic reaction pathway between the electron-deficient dienophile SO2 and the electron-rich diene o-xylylene are compared by optimization of the corresponding reactants, transition states and products at the PM6 level. Links to the calculation output for the optimized structures of o-xylylene, [30] SO2, [31] the exo,[32] and endo[33] product for the expected Diels-Alder reaction, the cheletropic product,[34] the exo, [35] and endo product [36] for the alternative Diels-Alder reaction, the exo [37] and endo [38] transition state for the expected Diels-Alder reaction, the cheletropic transition state,[39] the exo[40] and endo[41] transition states for the alternative Diels-Alder reaction, and the intrinsic reaction coordinate (IRC) of the exo[42] and endo[43] pathways of the expected Diels-Alder reaction, and the cheletropic[44] pathway can be found in References.
An analysis of reactant FMO overlap has not been included in this Exercise: it is expected that similar overlaps exist here between the archetypal diene fragment and dienophile fragments, and secondary orbital and/or steric interactions in the transition state can be used to rationalize relative activation energies and whether the endo or exo Diels-Alder adduct is kinetically favoured. It is also expected that this system behaves like a normal electron-demand Diels-Alder reaction, considering that the diene has electron-donating alkyl substituents, whereas the dienophile contains electronegative oxygen atoms and electron-withdrawing S=O functioncal groups.
Visualization of Reaction Coordinate
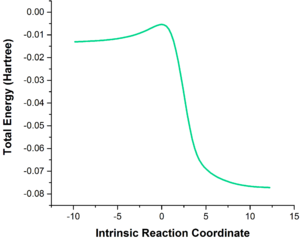
The IRCs were calculated and illustrated for the exo and endo pathways of the expected Diels-Alder reaction, and the cheletropic reaction. As shown in Table 6, the formation of two new bonds is asynchronous with both Diels-Alder reactions (with formation of the C-O bond slightly faster than that of the C-S bond), but synchronous in the cheletropic reaction (where both C-S bonds form simultaneously). In addition, the o-xylylene fragment appears to aromatize and form an aromatic 6-membered ring with delocalization extending to the carbon atoms outside the ring, which suggests that o-xylylene reacts as a diradical in the transition state. This would be due to the aromatic driving force and the inherent instability of o-xylylene.
Kinetics and Thermodynamics of Reaction Pathways
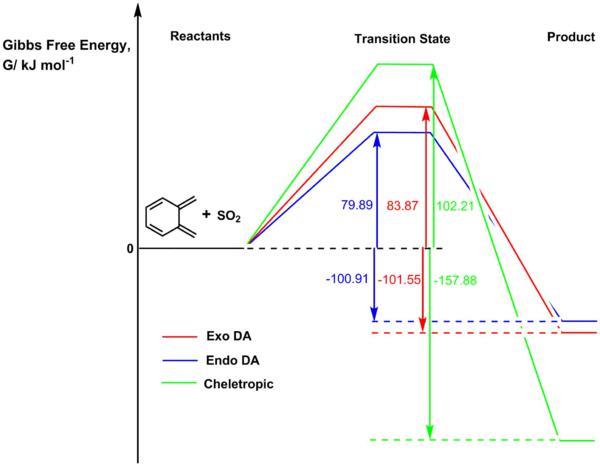
Analysis of activation energies and reaction energies (Table 7) revealed that for the expected Diels-Alder reaction, the endo product is the kinetic product and the exo product is the thermodynamic product, but overall, taking the chelotropic reaction into account, the chelotropic product is the thermodynamically favoured product (largest, most negative reaction energy) whereas the endo Diels-Alder product is the kinetic product (lowest activation energy). This suggests that if a mixture of o-xylylene and SO2 were allowed to react, the endo Diels-Alder product would be the fastest-formed, but if left to equilibrate, the product mixture will feature the cheletropic adduct as the major product; this result is consistent with literature observations and computations.[45] The reaction profile of all three pathways is illustrated in Figure 7.
| Computed Energy of Species (EH) | Activation Energy (kJ mol-1) | Reaction Energy (kJ mol-1) | ||
|---|---|---|---|---|
| Reactants | o-Xylylene | 0.178746 | N.A. | N.A. |
| SO2 | -0.118614 | |||
| Sum of Reactant Energies | 0.060132 | |||
| Transition State | Exo | 0.092077 | 83.87 | N.A. |
| Endo | 0.090559 | 79.89 | ||
| Cheletropic | 0.099062 | 102.21 | ||
| Product | Exo | 0.021454 | N.A. | -101.55 |
| Endo | 0.021696 | -100.91 | ||
| Cheletropic | -0.000001 | -157.88 | ||
 |
Diels-Alder Cycloaddition involving an Alternative Diene in o-Xylylene
As indicated in the Reaction Scheme, o-Xylylene contains a second diene fragment in an s-cis configuration, and the reactivity of this fragment in an alternative Diels-Alder reaction via the exo and endo pathways was examined by computation of the corresponding activation energies and reaction energies. As shown in Table 9, the kinetically favoured product is the endo product, and the thermodynamically favoured product is the exo product. However, the activation energies for both pathways are higher than their counterparts' in the previous Diels-Alder reaction, and the reaction enthalpies are both endothermic, as opposed to the exothermic reaction energies for the previous Diels-Alder reaction. Hence, this alternative Diels-Alder reaction is both kinetically and thermodynamically disfavoured with respect to the previous one discussed.
| Computed Energy of Species (EH) | Activation Energy (kJ mol-1) | Reaction Energy (kJ mol-1) | ||
|---|---|---|---|---|
| Transition State | Exo | 0.105057 | 117.95 | N.A. |
| Endo | 0.102071 | 110.11 | ||
| Product | Exo | 0.067304 | N.A. | 18.83 |
| Endo | 0.065609 | 14.38 | ||
Conclusion
In conclusion, the reactivity of the Diels-Alder reactions between the diene/dienophile systems, butadiene/ethene (Exercise 1), cyclohexadiene/1,3-dioxole (Exercise 2), and o-xylylene/SO2 (Exercise 3), were investigated via calculations on Gaussian software, and the reactants, transition state structures and products were optimized at PM6 (Exercise 1 and 3) and B3LYP/6-31G(d) (Exercise 2) levels of theory. IRC calculations were used to illustrate the reaction coordinate, and the type of Diels-Alder reaction (normal or inverse electron-demand) was determined from analysis of overlapping reactant FMOs. The activation energies and reaction energies were calculated to establish the kinetically and thermodynamically favoured reaction pathways, and the involvement of secondary orbital interactions and sterics was used to explain the lower activation energy for the formation of the kinetically favoured endo product for the cyclohexadiene/1,3-dioxole system (Exercise 2). Finally, optimized transition state structures with different sites of reaction were obtained, and used to determine regioselectivity in a system with more than one diene functional group (Exercise 3).
References
- ↑ 1.0 1.1 1.2 P. Pechukas, "Transition state theory.", Annual Review of Physical Chemistry, 1981, 32, 159-177.DOI:10.1146/annurev.pc.32.100181.001111
- ↑ "2-Dimensional Potential Energy Surface." Taken from: http://211.81.50.59//education/OCPkb/module2.php
- ↑ A. D. Becke, "A new mixing of Hartree–Fock and local density‐functional theories", J. Chem. Phy., 1993, 98, 1372.DOI:10.1063/1.464304
- ↑ "PM6-optimized Structure of Butadiene." https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:WLT_EXERCISE_1_BUTADIENE_MOS_OPT.LOG
- ↑ "PM6-optimized Structure of Ethene." https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:WLT_EXERCISE_1_ETHENE_MOS_OPT.LOG
- ↑ "PM6-optimized Structure of Cyclohexene." https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:WLT_EXERCISE_1_CYCLOHEXENE_OPT.LOG
- ↑ "PM6-optimized Transition State Structure for Exercise 1." https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:WLT_EXERCISE_1_CYCLOHEXENE_FROZEN_TS_MOS_OPT.LOG
- ↑ "Intrinsic Reaction Coordinate (IRC) for Exercise 1." https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:WLT_EXERCISE_1_CYCLOHEXENE_FROZEN_TS_IRC.LOG
- ↑ K. Fukui, T. Yonezawa, and H. Shingu, "Molecular Orbital Theory of Reactivity in Aromatic Hydrocarbons", J. Chem. Phy., 1952, 20, 722.DOI:10.1063/1.1700243
- ↑ F. A. Carey, R. J. Sundberg. (2007). "Advanced Organic Chemistry: Part A: Structures and Mechanisms (5th ed.)", New York: Springer. ISBN 978-0-387-44899-2
- ↑ G. Hilt, K. I. Smolko, B. V. Lotsch, "Cobalt(I)-catalyzed Neutral Diels-Alder Reactions of Oxygen-functionalized Acyclic 1,3-Dienes with Alkynes", Synlett, 2002, 7, 1081-1084.DOI:10.1063/1.1700243
- ↑ 12.0 12.1 12.2 12.3 J. Clayden, N. Greeves, S. Warren, P. Wothers. (2001). "Organic Chemistry (2nd ed.)", OUP Oxford. ISBN 978-0-19-927029-3
- ↑ S. S. Batsanov. "Van der Waals Radii of Elements", Inorganic Materials, 2001, 37, 871-885.DOI:10.1023/A:1011625728803
- ↑ "PM6-optimized Structure of Cyclohexadiene." https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:WLT_EXERCISE_2_CYCLOHEXADIENE_REACTANT_SYMMETRY_BROKEN.LOG
- ↑ "B3LYP/6-31G(d)-optimized Structure of Cyclohexadiene." https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:WLT_EXERCISE_2_CYCLOHEXADIENE_REACTANT_SYMMETRY_BROKEN_B3LYP_6-31G(D)_OPT.LOG
- ↑ "PM6-optimized Structure of 1,3-Dioxole." https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:WLT_EXERCISE_2_DIOXOLE_REACTANT_OPT.LOG
- ↑ "B3LYP/6-31G(d)-optimized Structure of 1,3-dioxole." https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:WLT_EXERCISE_2_DIOXOLE_REACTANT_SYMMETRY_BROKEN_B3LYP_6-31G(D)_OPT.LOG
- ↑ "PM6-optimized Structure of the Exo Product for Exercise 2." https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:WLT_EXERCISE_2_EXO_PRODUCT_OPT.LOG
- ↑ "B3LYP/6-31G(d)-optimized Structure of the Exo Product for Exercise 2." https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:WLT_EXERCISE_2_EXO_PRODUCT_B3LYP_6-31G(D)_OPT.LOG
- ↑ "PM6-optimized Structure of the Endo Product for Exercise 2." https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:WLT_EXERCISE_2_ENDO_PRODUCT_OPT.LOG
- ↑ "B3LYP/6-31G(d)-optimized Structure of the Endo Product for Exercise 2." https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:WLT_EXERCISE_2_ENDO_PRODUCT_B3LYP_6-31G(D)_OPT.LOG
- ↑ "PM6-optimized Exo Transition State Structure for Exercise 2." https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:WLT_EXERCISE_2_EXO_PRODUCT_FROZEN_TS_OPT.LOG
- ↑ "B3LYP/6-31G(d)-optimized Exo Transition State Structure for Exercise 2." https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:Wlt_exercise_2_exo_product_frozen_TS_B3LYP_6-31G(d)_MO_opt.log
- ↑ "PM6-optimized Endo Transition State Structure for Exercise 2." https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:WLT_EXERCISE_2_ENDO_PRODUCT_FROZEN_TS_OPT.LOG
- ↑ "B3LYP/6-31G(d)-optimized Endo Transition State Structure for Exercise 2." https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:Wlt_exercise_2_endo_product_frozen_TS_B3LYP_6-31G(d)_MO_opt.log
- ↑ "PM6-optimized Intrinsic Reaction Coordinate (IRC) for Exo Transition State in Exercise 2." https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:WLT_EXERCISE_2_EXO_PRODUCT_FROZEN_TS_IRC_RECORRECT_NEVER.LOG
- ↑ "B3LYP/6-31G(d)-optimized Intrinsic Reaction Coordinate (IRC) for Exo Transition State in Exercise 2." https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:WLT_exercise_2_exo_product_frozen_TS_B3LYP_6-31G(d)_IRC.log
- ↑ "PM6-optimized Intrinsic Reaction Coordinate (IRC) for the Endo Transition State in Exercise 2." https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:WLT_EXERCISE_2_ENDO_PRODUCT_FROZEN_TS_IRC.LOG
- ↑ "B3LYP/6-31G(d)-optimized Intrinsic Reaction Coordinate (IRC) for the Endo Transition State in Exercise 2." https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:WLT_exercise_2_endo_product_frozen_TS_B3LYP_6-31G(d)_IRC.log
- ↑ "PM6-optimized Structure of o-Xylylene." https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:WLT_EXERCISE_3_DIENE_REACTANT_OPT.LOG
- ↑ "PM6-optimized Structure of Sulfur Dioxide." https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:WLT_EXERCISE_3_SO2_REACTANT_OPT.LOG
- ↑ "PM6-optimized Structure of the Exo Product from the Expected Diels-Alder Reaction for Exercise 3." https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:WLT_TUTORIAL_METHOD_3_XYLYLENE-SO2_OPT.LOG
- ↑ "PM6-optimized Structure of the Endo Product from the Expected Diels-Alder Reaction for Exercise 3." https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:WLT_EXERCISE_3_ENDO_PRODUCT_OPT.LOG
- ↑ "PM6-optimized Structure of the Cheletropic Product for Exercise 3." https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:WLT_EXERCISE_3_CHELETROPIC_PRODUCT_OPT.LOG
- ↑ "PM6-optimized Structure of the Exo Product from the Alternative Diels-Alder Reaction for Exercise 3." https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:WLT_EXERCISE_3_OTHER_DA_EXO_PRODUCT_OPT.LOG
- ↑ "PM6-optimized Structure of the Endo Product from the Alternative Diels-Alder Reaction for Exercise 3." https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:WLT_EXERCISE_3_OTHER_DA_ENDO_PRODUCT_OPT.LOG
- ↑ "PM6-optimized Transition State Structure of the Exo Pathway of the Expected Diels-Alder Reaction for Exercise 3." https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:WLT_TUTORIAL_METHOD_3_XYLYLENE-SO2_FROZEN_TS_OPT.LOG
- ↑ "PM6-optimized Transition State Structure of the Endo Pathway of the Expected Diels-Alder Reaction for Exercise 3." https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:WLT_XYLELENE-SO2_ENDO_POSTFROZEN_TS_OPT.LOG
- ↑ "PM6-optimized Transition State Structure of the Cheletropic Pathway of the Expected Diels-Alder Reaction for Exercise 3." https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:WLT_EXERCISE_3_CHELETROPIC_FROZEN_TS_OPT.LOG
- ↑ "PM6-optimized Exo Transition State Structure of the Alternative Diels-Alder Reaction for Exercise 3." https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:WLT_EXERCISE_3_OTHER_DA_EXO_POSTFROZEN_TS_OPT.LOG
- ↑ "PM6-optimized Endo Transition State Structure of the Alternative Diels-Alder Reaction for Exercise 3." https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:WLT_EXERCISE_3_OTHER_DA_ENDO_POSTFROZEN_TS_OPT.LOG
- ↑ "Intrinsic Reaction Coordinate (IRC) of the Exo Pathway of the Expected Diels-Alder Reaction for Exercise 3." https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:WLT_TUTORIAL_METHOD_3_XYLYLENE_SO2_TS_IRC.LOG
- ↑ "Intrinsic Reaction Coordinate (IRC) of the Exo Pathway of the Expected Diels-Alder Reaction for Exercise 3." https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:WLT_XYLELENE-SO2_ENDO_POSTFROZEN_TS_IRC.LOG
- ↑ "Intrinsic Reaction Coordinate (IRC) of the Cheletropic Reaction Pathway for Exercise 3." https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:WLT_EXERCISE_3_CHELETROPIC_FROZEN_TS_IRC.LOG
- ↑ D. Suarez, T. L. Sordo, J. A. Sordo, "A Comparative Analysis of the Mechanisms of Cheletropic and Diels-Alder Reactions of 1,3-Dienes with Sulfur Dioxide: Kinetic and Thermodynamic Controls", J. Org. Chem., 1995, 60, 2848-2852.DOI:10.1021/jo00114a039