Rep:Mod:WMX2012molecule3
The Cope Rearrangement
Optimizing the Reactants and Products
1,5-hexadiene with an "anti" linkage
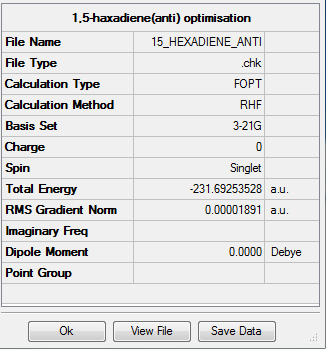
The "anti" 1,5-hexadiene was optimized at the HF/3-21G level of theory with memory limit of 250MB. The followings are the summary, symmetry group and molecule structure of the structure:
Molecule
"Anti" 1,5-hexadiene molecule |
Summary
Output information
Item Value Threshold Converged? Maximum Force 0.000060 0.000450 YES RMS Force 0.000010 0.000300 YES Maximum Displacement 0.000442 0.001800 YES RMS Displacement 0.000171 0.001200 YES
Symmetry group
File Link
File:WENMIN 15 HEXADIENE ANTI.LOG
1,5-hexadiene with an "gauche" linkage
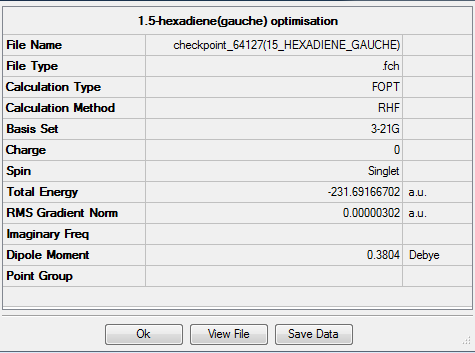
The "gauche" form of 1,5-hexadiene should have lower energy than the "anti" one. The "gauche" 1,5-hexadiene was optimized at the HF/3-21G level of theory with memory limit of 250MB. The followings are the summary and symmetry of the structure:
Molecule
"Gauche" 1,5-hexadiene molecule |
Summary
Output information
Item Value Threshold Converged? Maximum Force 0.000008 0.000450 YES RMS Force 0.000003 0.000300 YES Maximum Displacement 0.000174 0.001800 YES RMS Displacement 0.000055 0.001200 YES
Symmetry group
File Link
File:WENMIN Log 64127(15 HEXADIENE GAUCHE).log
Lowest energy conformation
The lowest energy conformation should be the gauche3 form (shown in the Appendix 1[1]). This form is drawn and optimized at the HF/3-21G level with memory limit of 250MB. The followings are the results:
Molecule
1,5-hexadiene molecule with lowest energy |
Summary
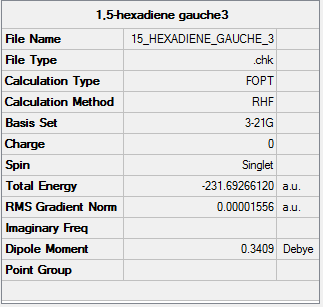
Output information
Item Value Threshold Converged? Maximum Force 0.000044 0.000450 YES RMS Force 0.000009 0.000300 YES Maximum Displacement 0.001316 0.001800 YES RMS Displacement 0.000491 0.001200 YES
Symmetry group
File Link
File:WENMIN 15 HEXADIENE GAUCHE 3.LOG
Structure table
Structures and energies are compared with the reference[2], conformers are identified with specific names.
| Conformer | Structure | Point Group | Energy/Hartrees HF/3-21G |
| Section 1.1.2: gauche2 | C2 | -231.69166702 | |
| Section 1.1.3: gauche3 | C1 | -231.69266120 | |
| Section 1.1.1: anti2 | Ci | -231.69253528 |
From the table shown above, we get that gauche3 conformation has the lowest energy, followed by anti2 conformation. The gauche2 conformation has the highest energy here.
We usually have the impression that anti2 conformation should be lowest in energy level for allyl groups. This is mainly because, for small allyl molecules, the anti-periplanar form has the maximizing σC-H/σ*C-H and/or σC-C/σ*C-C conjugation. In addition, the app form relieves the torsional strain. However, as the allyl molecule number increases, gauche form becomes promoted. This is due to the longer distances favoring van der Waals (dispersion) interaction by folding chain back upon itself. In this case, alkene group results in fewer H groups on the sp2 carbon and some bond angles are wider. This leads to reduce hindrance and avoid the H...H contacts of smaller than 2.1Å at which point hydrogen becomes repulsive. What's more, better overlap of πC=C/σ*C=C results in larger stabilizing energy.
Reoptimization of "anti2" conformation of 1,5-hexadiene
The "anti2" conformation is reoptimized at the B3LYP/6-31G(d) level. The followings are the results:
Molecule
"Anti2" 1,5-hexadiene molecule |
Summary
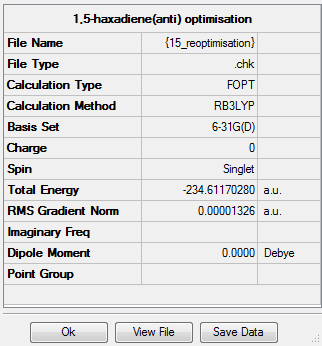
Output information
Item Value Threshold Converged? Maximum Force 0.000015 0.000450 YES RMS Force 0.000006 0.000300 YES Maximum Displacement 0.000219 0.001800 YES RMS Displacement 0.000079 0.001200 YES
Symmetry group
The symmetry group changes to C2h at the B3LYP/6-31G(d) level from Ci at the HF/3-21G level.
File Link
File:WENMIN 15 HEXADIENE ANTI REOPT.LOG
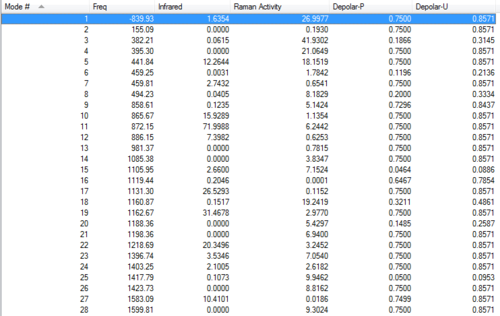
Frequency of "anti2" conformation of 1,5-hexadiene
The frequency of the "anti2" conformation is calculated at the B3LYP/6-31G(d) level. The followings are the results:
Summary

Frequencies
All is real frequencies, no imaginary ones.

Energy lists
Sum of electronic and zero-point Energies= -234.469212 Sum of electronic and thermal Energies= -234.461856 Sum of electronic and thermal Enthalpies= -234.460912 Sum of electronic and thermal Free Energies= -234.500821
IR

File Link
File:WENMIN 15 HEXADIENE ANTI FREQ.LOG
Optimizing the "Chair" and "Boat" Transition
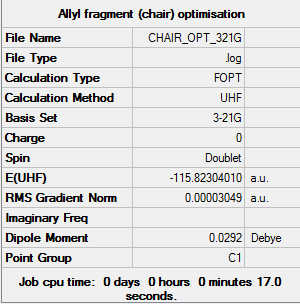
Allyl fragment optimization
The allyl fragment (CH2CHCH2) is optimized at the HF/3-31G level of theory. The followings are the results:
Molecule
Allyl fragment |
Summary
Output information
Item Value Threshold Converged? Maximum Force 0.000048 0.000450 YES RMS Force 0.000018 0.000300 YES Maximum Displacement 0.000141 0.001800 YES RMS Displacement 0.000070 0.001200 YES
File Link
File:WENMIN CHAIR OPT 321G.LOG
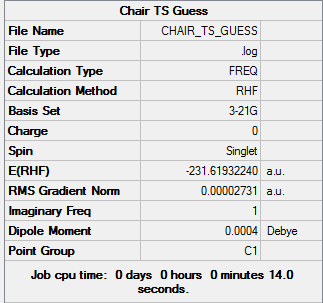
Guess TS optimization and frequency
The guess TS is made from two allyl fragments with approximately 2.2Å terminal ends. Then the guess TS is calculated under Opt+Freq job type, with Optimization to a TS (Berny), calculate the force constants Once, and type Opt=NoEigen in the Additional keyword box. The followings are the results:
Molecule
Guess TS |
Summary
Output information
Item Value Threshold Converged? Maximum Force 0.000024 0.000450 YES RMS Force 0.000006 0.000300 YES Maximum Displacement 0.000829 0.001800 YES RMS Displacement 0.000115 0.001200 YES
Vibration frequencies
There's only one imaginary frequency of magnitude 817.98 cm-1, conforming that this geometry is a TS.

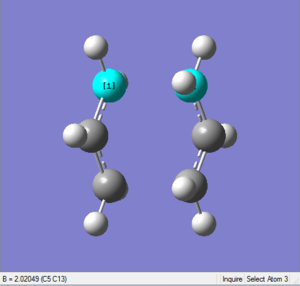
Bond distance
The bond distances of terminal ends change to about 2.02Å, as shown in the following two diagrams.

File Link
File:WENMIN CHAIR TS GUESS.LOG
Guess TS optimization using the frozen coordinate method
The guess TS is optimized at the HF/3-31G level of theory, with minimum optimization and the Freeze Coordinate under Bond. However, the Gaussian program doesn't respond to the input of 2.20Å fixed bond length. The result obtained does not have 2.20Å terminal ends although they are very close to 2.20Å.


So I redo the calculation, but this time with fixed 2.20Å terminal ends using "Modify Bond" tool. The followings are the results of second trail:
Molecule
Guess TS with frozen coordinate |
Summary
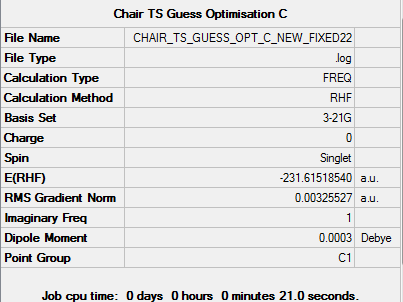
Output information
Item Value Threshold Converged? Maximum Force 0.000010 0.000450 YES RMS Force 0.000003 0.000300 YES Maximum Displacement 0.001213 0.001800 YES RMS Displacement 0.000247 0.001200 YES
Vibration frequencies
There's only one imaginary frequency, conforming that this geometry is a TS.
Bond distance
This time the length between two terminal ends is exactly 2.20Å, as shown in the following two diagrams.
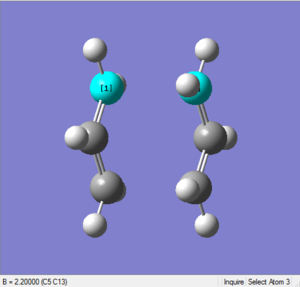
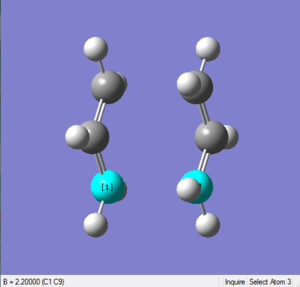
File Link
File:WENMIN CHAIR TS GUESS OPT C NEW FIXED22.LOG
Guess TS optimization using the derivative coordinate method
The guess TS obtained from the frozen coordinate method is optimized at the HF/3-31G level of theory, with minimum optimization and the Derivative under Bond. The followings are the results:
Molecule
Guess TS with derivative coordinate |
Summary

Output information
Item Value Threshold Converged? Maximum Force 0.000032 0.000450 YES RMS Force 0.000009 0.000300 YES Maximum Displacement 0.001154 0.001800 YES RMS Displacement 0.000230 0.001200 YES
Vibration frequencies
There's only one imaginary frequency, conforming that this geometry is a TS. The magnitude of the imaginary frequency is 818.06 cm-1, which is very close to the one got from section 1.2.2.
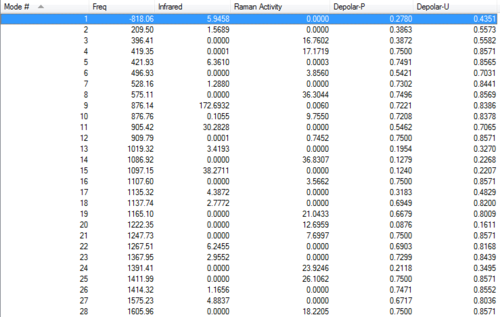
Energy list
Sum of electronic and zero-point Energies= -231.466693 Sum of electronic and thermal Energies= -231.461334 Sum of electronic and thermal Enthalpies= -231.460390 Sum of electronic and thermal Free Energies= -231.495198
Bond distance
The bond distances of terminal ends change to about 2.02Å, as shown in the following two diagrams. When comparing the structures obtained from section 1.2.2 and this section, they are almost identical, both having approximate 2.02Å bond distances between terminal ends. This shows that both methods are good approach to determine the TS, since this is a pretty good "guess" of the transition state structure.


File Link
File:WENMIN CHAIR TS GUESS OPT D.LOG
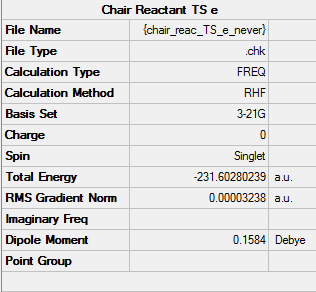
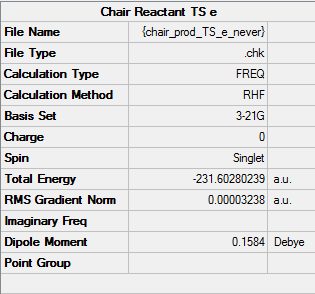
Boat TS optimization using the QST2 method
The reactant and product are calculated under Opt+Freq job type with Optimization to a TS (QST2), using the structures shown as below.

The followings are the results:
Molecule
Reactant |
Product |
Summary
NOTE: Ignore the "Chair" under file name. These are boat structures.
Output information
Reactant:
Item Value Threshold Converged? Maximum Force 0.000105 0.000450 YES RMS Force 0.000022 0.000300 YES Maximum Displacement 0.001249 0.001800 YES RMS Displacement 0.000277 0.001200 YES
Product:
Item Value Threshold Converged? Maximum Force 0.000105 0.000450 YES RMS Force 0.000022 0.000300 YES Maximum Displacement 0.001249 0.001800 YES RMS Displacement 0.000277 0.001200 YES
Energy list
Reactant:
Sum of electronic and zero-point Energies= -231.450922 Sum of electronic and thermal Energies= -231.445294 Sum of electronic and thermal Enthalpies= -231.444349 Sum of electronic and thermal Free Energies= -231.479768
Product:
Sum of electronic and zero-point Energies= -231.450922 Sum of electronic and thermal Energies= -231.445294 Sum of electronic and thermal Enthalpies= -231.444349 Sum of electronic and thermal Free Energies= -231.479768
Vibration frequencies
There's only one imaginary frequency, conforming that this geometry is a TS (for reactant and product approach).
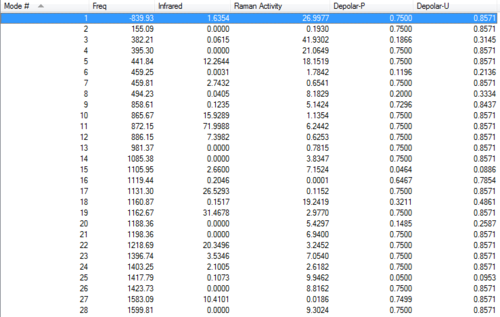
Since the structure of reactant and product is exactly the same, all results are the same.
File Link
Reactant: File:WENMIN CHAIR REAC TS E.LOG
Product: File:WENMIN CHAIR PROD TS E.LOG
Chair TS IRC
The IRC (Intrinsic Reaction Coordinate) method is used to follow the minimum energy path from a transition structure down its local minimum on a potential energy surface. The optimized chair TS (with derivative bond in this case) is calculated at HF/3-21 level with IRC under the Job Type tab. Compute it in the forward direction, calculate the force constants once and change the number of points along the IRC into 50.
IRC result

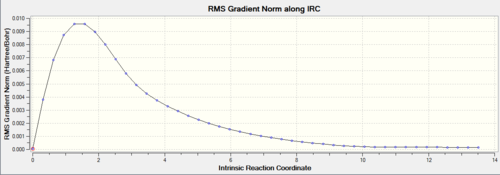
44 intermediate geometries are obtained and the last one has the energy of -231.69157861 a.u.
Options to reach minimum geometry
Then three options are tested receptively to get a minimum conformation.
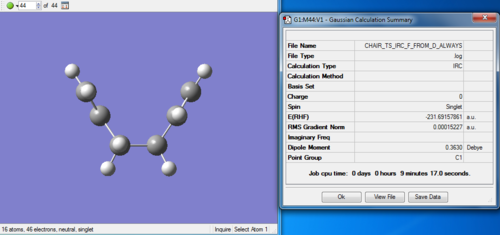
(i) The last point on the IRC is taken to do a minimum optimization and the energy obtained is -231.69166702 a.u, which is lower than -231.69157861 a.u.

(ii) The chair TS is run an IRC again, but this time with 100 points instead of 50. However, the same result is obtained as the original one (50 points), having 44 intermediate geometries with minimum energy of -231.69157861 a.u. (I try to run this with additional note of maxcycles=50, but the result is unchanged.)

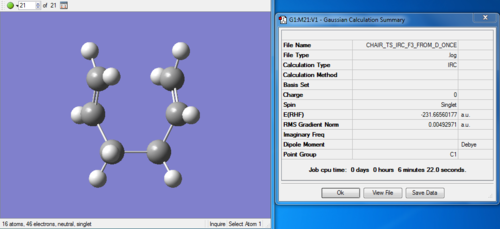
(iii) Redo the IRC specifying that compute the force constant at every step. The job is incomplete, resulting only 21 intermediate geometries.
Overall, the step (i) is the only approach actually works, ending up with the conformation of energy -231.69166702 a.u.
File link
IRC: File:WENMIN CHAIR TS IRC F FROM D ALWAYS.LOG
Optimization after IRC: File:WENMIN CHAIR TS IRC F1 FROM D OPT.LOG
Redo IRC with "n=100": File:WENMIN CHAIR TS IRC F2 FROM D ALWAYS.LOG
Redo IRC with "compute force constants at every step": File:WENMIN CHAIR TS IRC F3 FROM D ONCE.LOG
Boat & Chair TS reoptimization at higher level
The chair and boat transition structures are reoptimized using the B3LYP/6-31G(d) level of theory and carry out the frequency calculations.
Boat TS reoptimization at higher level
Molecule
Boat TS molecule |
Summary
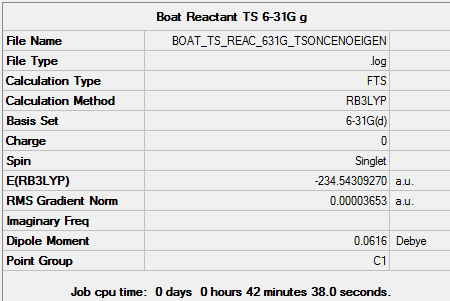
Output information
Item Value Threshold Converged? Maximum Force 0.000073 0.000450 YES RMS Force 0.000016 0.000300 YES Maximum Displacement 0.001276 0.001800 YES RMS Displacement 0.000316 0.001200 YES
Energy list
Sum of electronic and zero-point Energies= -234.402336 Sum of electronic and thermal Energies= -234.396003 Sum of electronic and thermal Enthalpies= -234.395059 Sum of electronic and thermal Free Energies= -234.431745
File Link
Reoptimization: File:WENMIN BOAT TS REAC 631G TSONCENOEIGEN.LOG
Frequency: File:WENMIN BOAT TS REAC 631G FREQ.LOG
Chair TS reoptimization at higher level
Molecule
Chair TS molecule |
Summary
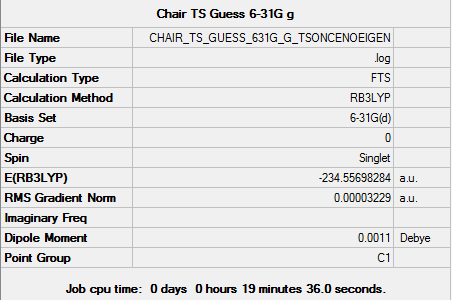
Output information
Item Value Threshold Converged? Maximum Force 0.000025 0.000450 YES RMS Force 0.000006 0.000300 YES Maximum Displacement 0.001476 0.001800 YES RMS Displacement 0.000214 0.001200 YES
Energy list
Sum of electronic and zero-point Energies= -234.414929 Sum of electronic and thermal Energies= -234.409008 Sum of electronic and thermal Enthalpies= -234.408064 Sum of electronic and thermal Free Energies= -234.443814
File Link
Reoptimization: File:WENMIN CHAIR TS GUESS 631G G TSONCENOEIGEN.LOG
Frequency: File:WENMIN CHAIR TS GUESS 631G G FREQ.LOG
Results Table
Summary of energies (in hartree)
| HF/3-21G | B3LYP/6-31G* | |||||
|---|---|---|---|---|---|---|
| Electronic energy | Sum of electronic and zero-point energies | Sum of electronic and thermal energies | Electronic energy | Sum of electronic and zero-point energies | Sum of electronic and thermal energies | |
| at 0 K | at 298.15 K | at 0 K | at 298.15 K | |||
| Chair TS | -231.619322 | -231.466693 | -231.461334 | -234.556983 | -234.414929 | -234.409008 |
| Boat TS | -231.602802 | -231.450922 | -231.445294 | -234.543093 | -234.402336 | -234.396003 |
| Reactant (anti2) | -231.692535 | -231.539539 | -231.532565 | -234.611703 | -234.469212 | -234.461856 |
*1 hartree = 627.509 kcal/mol
Summary of activation energies (in kcal/mol)
| HF/3-21G | HF/3-21G | B3LYP/6-31G* | B3LYP/6-31G* | Expt.[3] | |
| at 0 K | at 298.15 K | at 0 K | at 298.15 K | at 0 K | |
| ΔE (Chair) | 45.71 | 44.70 | 34.06 | 33.16 | 33.5 ± 0.5 |
| ΔE (Boat) | 55.61 | 54.76 | 41.97 | 41.32 | 44.7 ± 2.0 |
When comparing the TS energy at higher level (B3LYP/6-31G*) with the one at lower level (HF/3-21G), the difference is not very obvious in hartree unit. But even 0.01 difference in hartree means about 6 kcal/mol difference. This can be confirmed by the second table. The values obtained at B3LYP/6-31G* level agree with the experimental value much better than the ones at HF/3-21G since the HF method ignores the effect of electron correlation. Therefore, the energy obtained from HF method is systematically higher because the correlation energy of the TS is much greater. The activation energy of chair TS is lower than that of boat TS, so we can confirm that the Cope Rearrangement has a chair transition state.
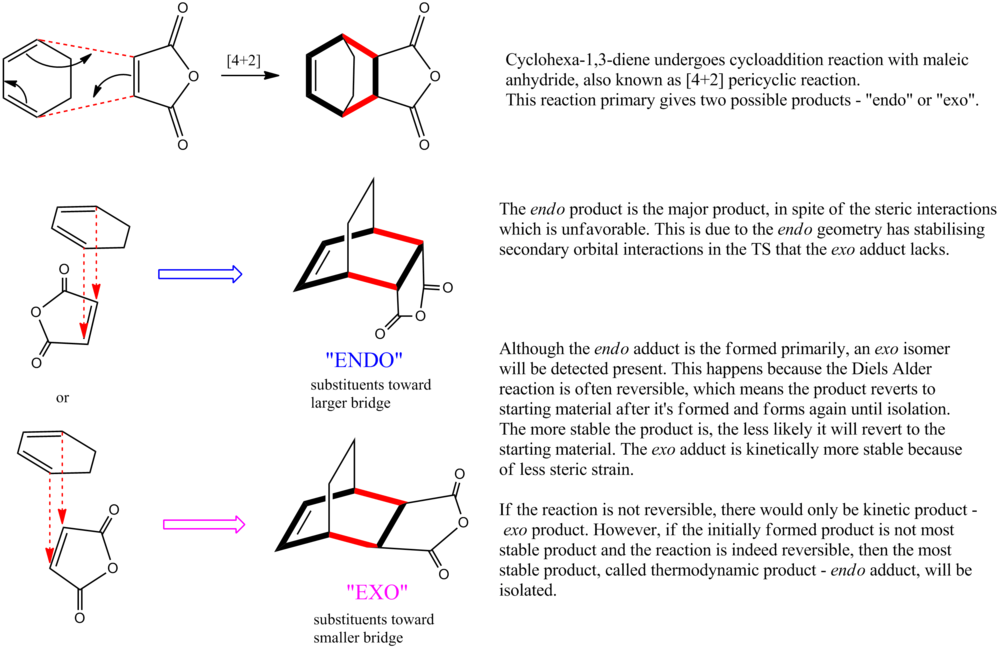
The Diels Alder Cycloaddition
In this section, all calculations are carried out using the AM1 semi-empirical molecular orbital method. As for the computation of the TS geometry optimization, TS (Berny), calculate the force constants Once, and additional keyword of Opt=NoEigen are specified because it's a better approach as shown in the first part.
Cis-butadiene
A cis-butadiene is drawn and optmized, using the AM1 semi-empirical molecular orbital method by the GaussView. The followings are the result:
Molecule
Cis-butadiene molecule |
Summary
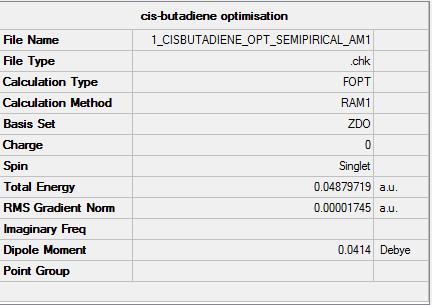
Output information
Item Value Threshold Converged? Maximum Force 0.000030 0.000450 YES RMS Force 0.000011 0.000300 YES Maximum Displacement 0.000330 0.001800 YES RMS Displacement 0.000162 0.001200 YES
HOMO and LUMO
| HOMO | LUMO |
|---|---|
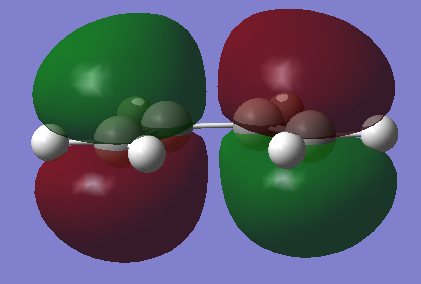 |
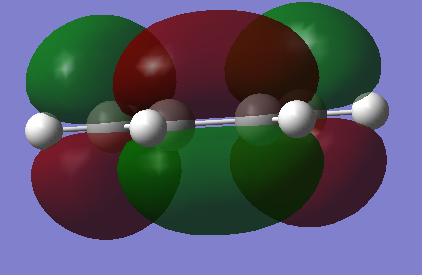 |
Link file
File:WENMIN 1 CISBUTADIENE OPT SEMIPIRICAL AM1.LOG
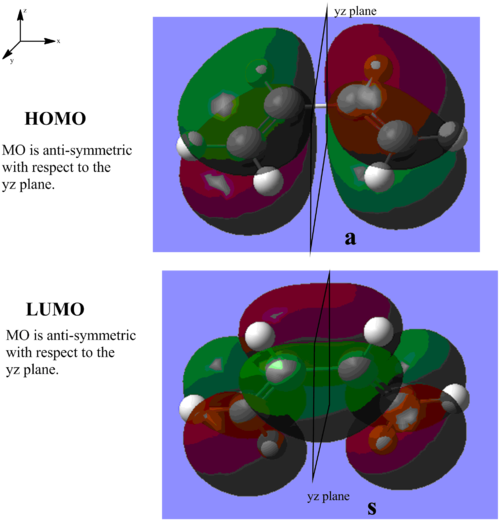
TS geometry for the prototype reaction
The guessing TS geometry for the prototype reaction is the geometry (a). It is optimized using TS (Berny), calculate the force constants Once, and additional keyword of Opt=NoEigen, under the AM1 semi-empirical molecular orbital method by the GaussView. The followings are the result:
Molecule
Guess TS |
Summary
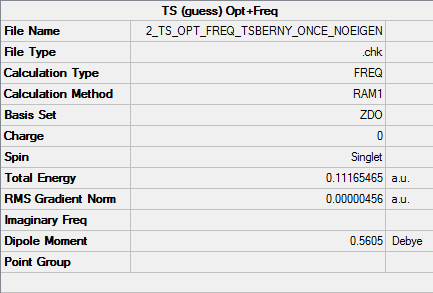
Output information
Item Value Threshold Converged? Maximum Force 0.000014 0.000450 YES RMS Force 0.000002 0.000300 YES Maximum Displacement 0.000080 0.001800 YES RMS Displacement 0.000026 0.001200 YES
Vibration frequencies
There's only one imaginary frequency of magnitude 956.19 cm-1, conforming that this geometry is a TS.
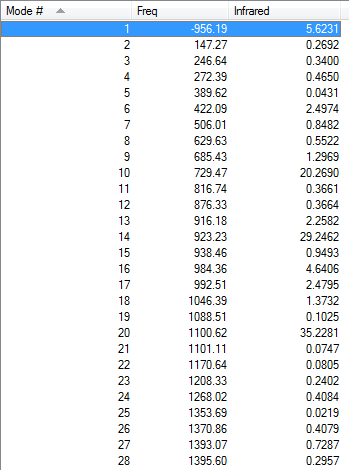
| Imaginary Frequency | Lowest Positive Frequency |
|---|---|
 |
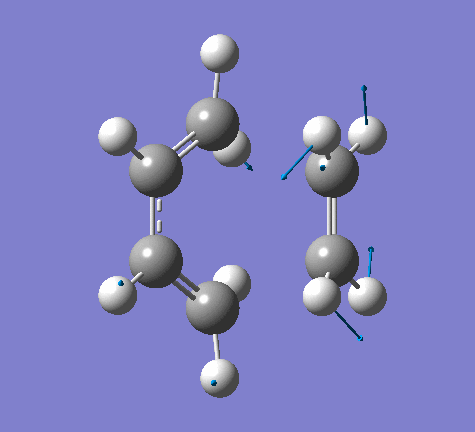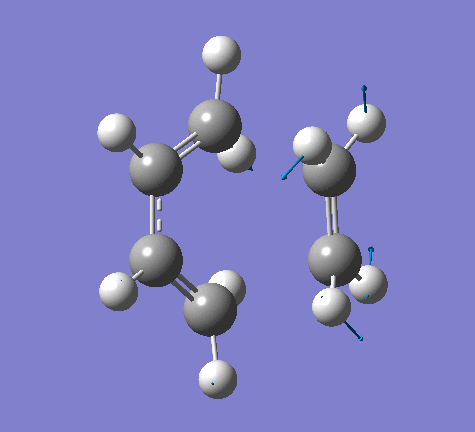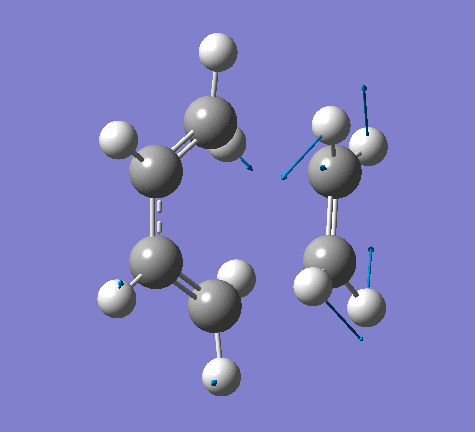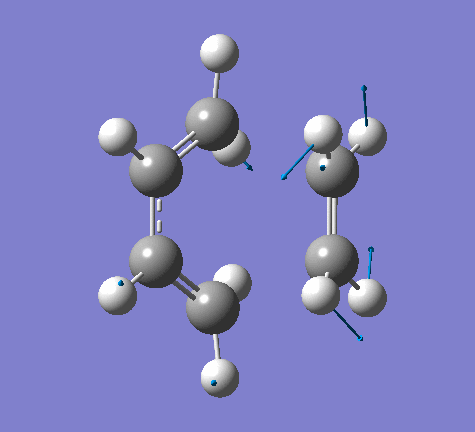
|
From the imaginary frequency we can see that the formation of two bonds is synchronous and symmetrical. This confirms that the concerted mechanisms of the reaction. The terminal ends of diene and ethylene are vibrating towards each other (back and forth). A reaction is undergoing between these two molecules. By contrast, in the lowest positive frequency, these two molecules are doing vibration on their own and no interaction whatsoever is happening between them. Another interesting point here is that carbon atoms and hydrogen atoms are vibrating in opposite directions in imaginary frequency, while C and H atoms are vibrating in the same direction in the lowest positive frequency.
HOMO & LUMO
| HOMO | LUMO |
|---|---|
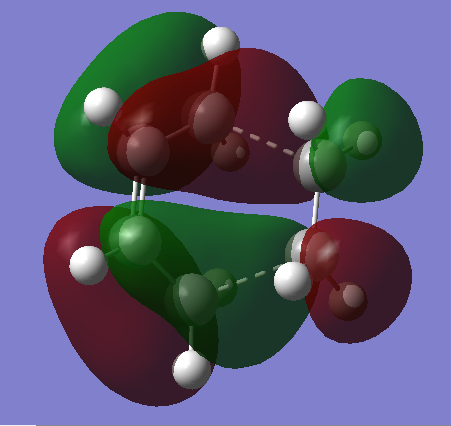 |
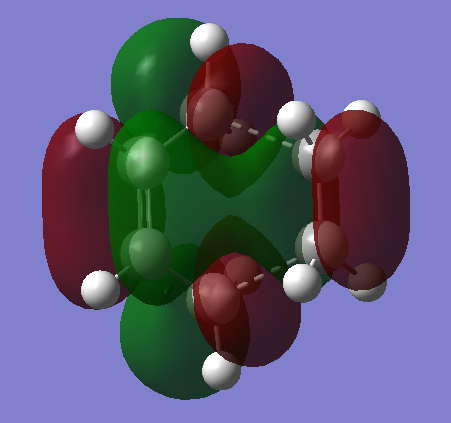 |

Bond length
 |
 |
| C-C sp3-sp3 | C-C sp2-sp2 | C-C van der Waals | C-C TS from calculation |
|---|---|---|---|
| 1.54Å[4] | 1.34Å[5] | ~1.7Å[6] | 2.12Å |
The bond distance in the TS, from calculation, is smaller than the van der Waals radius, but larger than that of both σC-C and πC-C bond. This shows the bond is partly formed into a σC-C bond during the transition state.
MO

Link file
File:WENMIN 2 TS OPT FREQ TSBERNY ONCE NOEIGEN.LOG
Regioselectivity of the Diels Alder Reaction
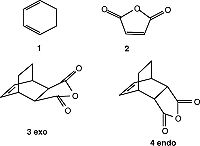
The reaction between 1 (cyclohexa-1,3-diene) and 2 (maleic anhydride) gives either the endo adduct primarily. This reaction is kinetically controlled so the exo TS should have higher energy than the endo TS. Both the exo and the endo TS are optimized using TS (Berny), calculate the force constants Once, and additional keyword of Opt=NoEigen, under the AM1 semi-empirical molecular orbital method by the GaussView.
Exo TS
Exo TS |
Summary
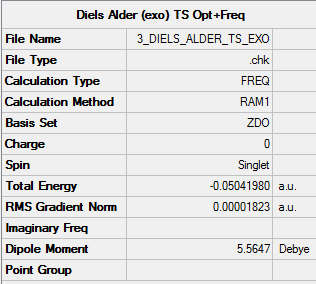
Output information
Item Value Threshold Converged? Maximum Force 0.000062 0.000450 YES RMS Force 0.000011 0.000300 YES Maximum Displacement 0.001361 0.001800 YES RMS Displacement 0.000195 0.001200 YES
Vibration frequencies
There's only one imaginary frequency of magnitude 812.38 cm-1, conforming that this geometry is a TS.
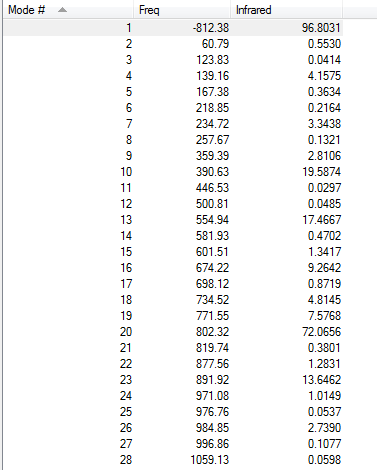
| Imaginary Frequency | Lowest Positive Frequency |
|---|---|
 |
|
As discussed in Section 2.2.4.
HOMO & LUMO
| HOMO | LUMO |
|---|---|
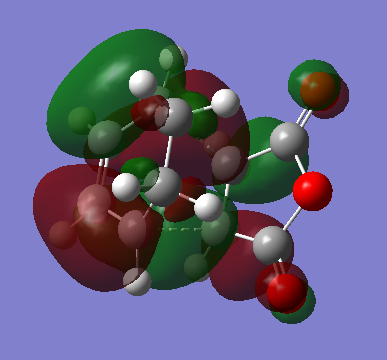 |
 |
Link file
File:WENMIN 3 DIELS ALDER TS EXO.LOG
Endo TS
Molecule
Endo TS |
Summary
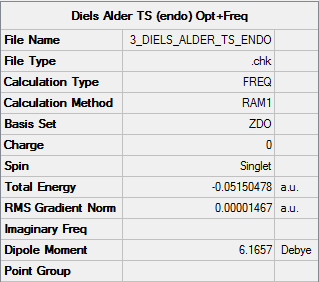
Output information
Item Value Threshold Converged? Maximum Force 0.000031 0.000450 YES RMS Force 0.000007 0.000300 YES Maximum Displacement 0.000757 0.001800 YES RMS Displacement 0.000128 0.001200 YES
Vibration frequencies
There's only one imaginary frequency of magnitude 806.22 cm-1, conforming that this geometry is a TS.
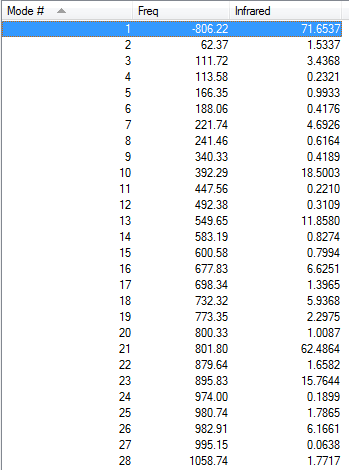
| Imaginary Frequency | Lowest Positive Frequency |
|---|---|
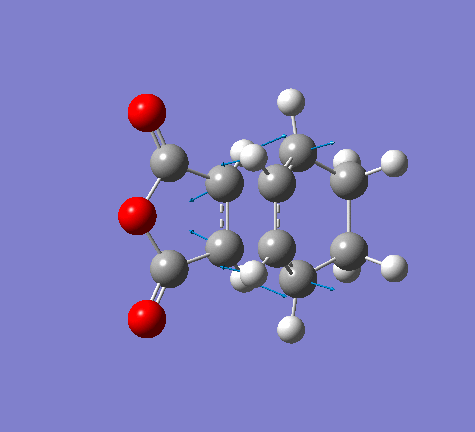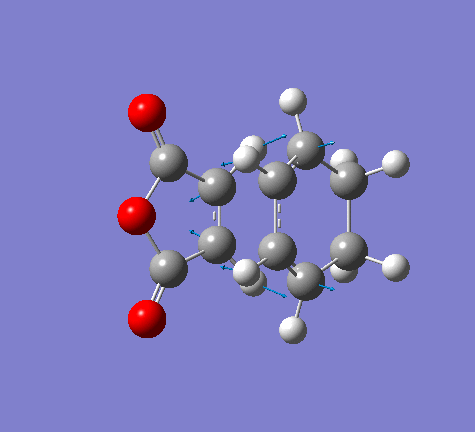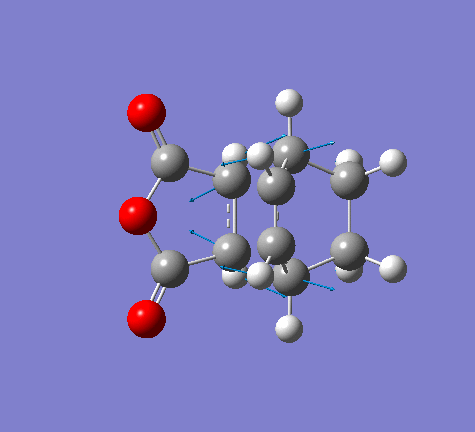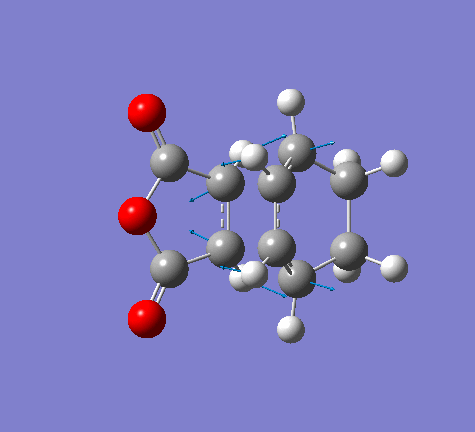 |
|
As discussed in Section 2.2.4.
HOMO & LUMO
| HOMO | LUMO |
|---|---|
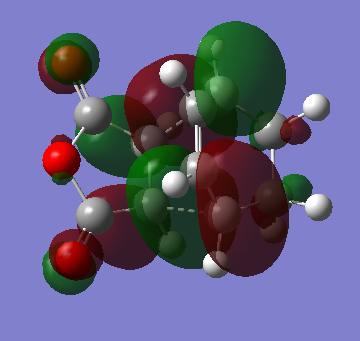 |
 |
Link file
File:WENMIN 3 DIELS ALDER TS ENDO.LOG
Explanation
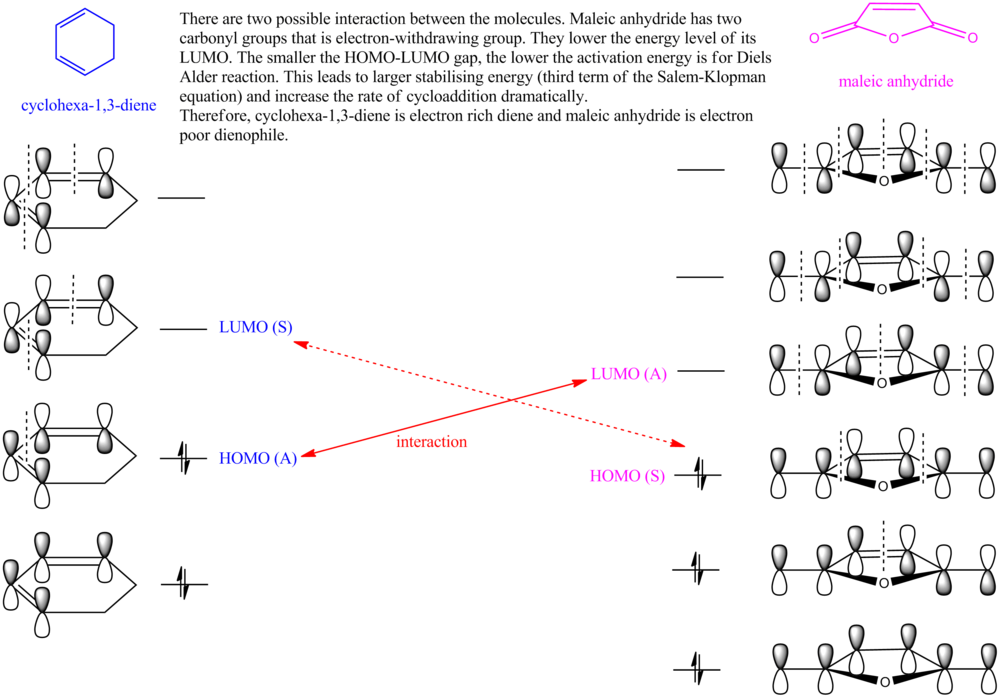
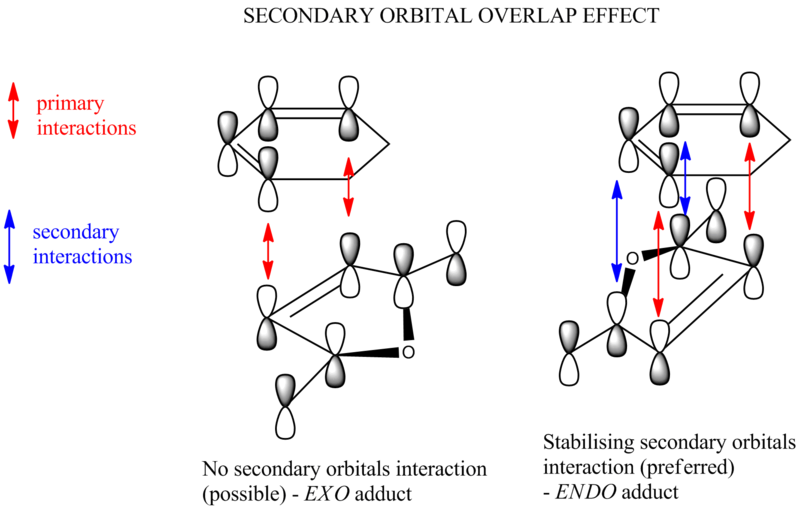
Now take a closer look at the MOs of HOMO from both exo and endo form. The endo form has strong overlap between the -(C=O)-O-(C=O)- fragment and the remainder of the system - secondary orbital interaction. This effect does not exist in the exo form.
| Exo | Endo |
|---|---|
 |

|
Comparison
| Exo | Endo |
|---|---|
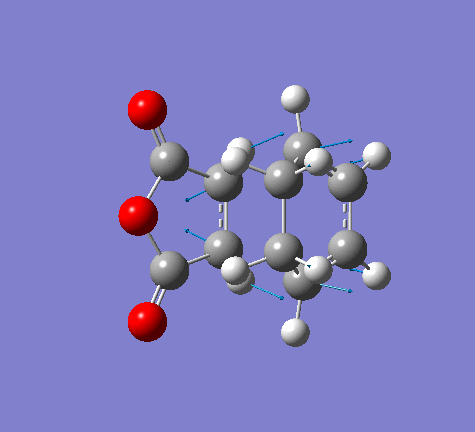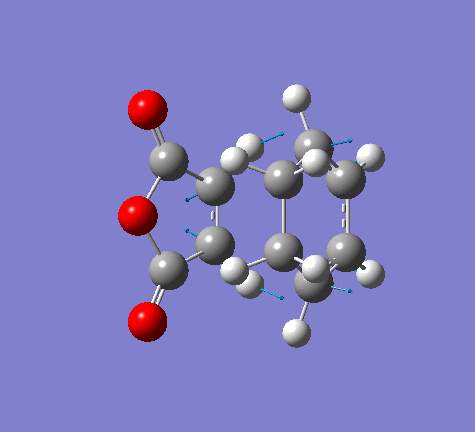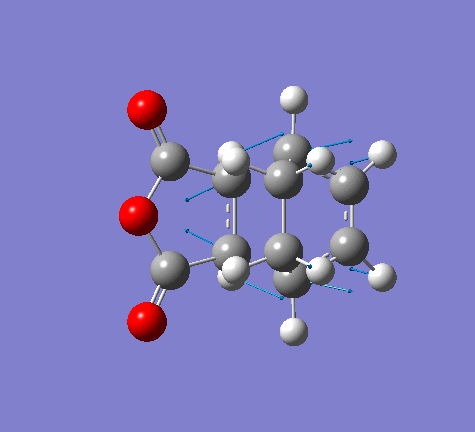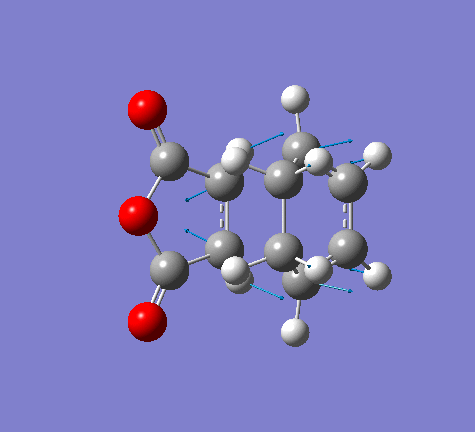 |
|
| Exo | Endo |
|---|---|
| -0.05041980 a.u. | -0.05150478 a.u. |
Energy difference ΔE = (-0.05041980) - (-0.05150478) = 1.08498*10-3 a.u. = 0.6808 kcal/mol
Endo geometry has lower energy level and thus it is favored.
The difference of partly formed C-C bond length in exo form and endo form is very small. The endo form has a little bit shorter length than the exo one since the endo form is the more stable.
| Exo | Endo |
|---|---|
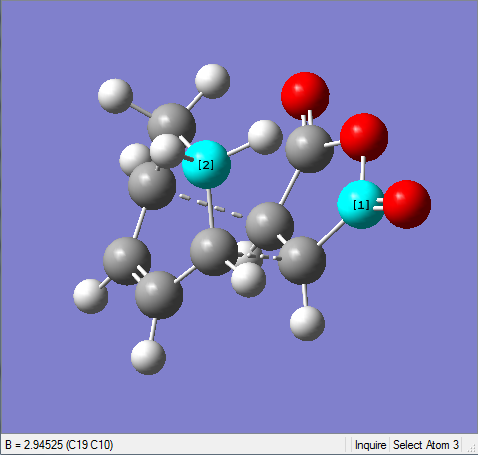 |
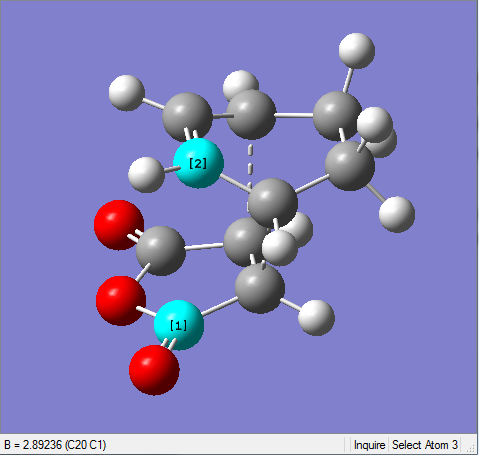
|
It's shown in the table above that the C-C space in exo TS is larger than the one in endo TS. This confirms that the endo form suffers more steric hindrance than the exo one. However, the difference between them is very small. This is due to the secondary orbital interaction in endo form that pulls carbonyl groups towards the diene, making the C-C space shorter than it should be. This is the result of secondary orbital interaction versus repulsion.
Further discussion
There's computational evidence showing that the semi-empirical calculation that neglects overlap inherently favors "ond-bond" (biradicaloid) TS for orbital symmetry allowed cycloadditions, whereas semi-empirical calculation that includes the overlap favor "two-bond" synchronous TS.[7] The calculations neglect the errors that may arise from anharmonicity of the vibrational potentials, deviations from transition-state theory can arise from tunneling, re-crossing and variational effects. In addition, the method totally ignores the solvent effects. Changing the solvent environment may result in favored exo form.
References
- ↑ Module 3: Transition states and reactivity. [[1]]
- ↑ Module 3: Transition states and reactivity. [[2]]
- ↑ Module 3: Transition states and reactivity. [[3]]
- ↑ 1986 J. Phys. C: Solid State Phys. 19 4613
- ↑ 1986 J. Phys. C: Solid State Phys. 19 4613
- ↑ Dr. Henry Zrepa, Conformational Analysis Lecture Notes [[4]]
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedsynchronous TS