Rep:Mod:WANGSIMENG
Week 1 Project: Optimisation and Analysis of BH3 and Other Simple Molecules
Wang, Simeng Year 3 Computational Lab Module 2
Basic Optimisation
This section contains the results obtained from the exercises in Day 1 and 2.
In this section, the result of basic optimisation of simple molecules, for instance BH3 and TlBr3, was presented and analysed. Method used in this section is 'B3LYP' and the basis sets used are '3-21G' and '6-31G'.
Optimisation of BH3 using 'B3LYP, 3-21G'
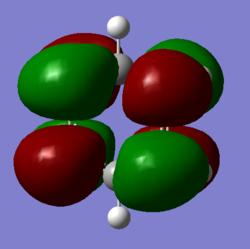
BH molecule |
BH3 molecule was first optimised with 3-21G basis set. This basic set has a lower level of accuracy but the calculation is very quick. The result obtained in this section will be further optimised with a better basis set in the next section.
Set-up
- Method: B3LYP
- Basis set: 3-21G
- Job Type: Optimisation
- Keywords: # opt b3lyp/3-21g geom=connectivity
Results
The log file of BH3 (opt 3-21G) is available here.
- Summary Table

| File type | .log |
|---|---|
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 3-21G |
| Final Energy | -26.46226338 a.u. |
| Gradient | 0.00020672 a.u. |
| Dipole Moment | 0.0000 Debye |
| Point Group | D3h |
| Calculation Time | 28.0 s |
- Item Table
Item Value Threshold Converged?
Maximum Force 0.000413 0.000450 YES
RMS Force 0.000271 0.000300 YES
Maximum Displacement 0.001610 0.001800 YES
RMS Displacement 0.001054 0.001200 YES
Predicted change in Energy=-1.071764D-06
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.1935 -DE/DX = 0.0004 !
! R2 R(1,3) 1.1935 -DE/DX = 0.0004 !
! R3 R(1,4) 1.1935 -DE/DX = 0.0004 !
! A1 A(2,1,3) 120.0 -DE/DX = 0.0 !
! A2 A(2,1,4) 120.0 -DE/DX = 0.0 !
! A3 A(3,1,4) 120.0 -DE/DX = 0.0 !
! D1 D(2,1,4,3) 180.0 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
Both the forces and the placements are converged.
- Other Results
B-H bond length = 1.19349 Å
H-B-H bond angle= 120
The length of B-H bonds and H-B-H bond angles are all equal, which is expected as the molecule is symmetrical and all the B-H bonds should have the same chemical environment.
Optimisation of BH3 using 'B3LYP, 6-31G'
After completing the optimisation of BH3 molecule using 3-21G basis set, the molecule was further optimised with 6-31G(d,p) basis set. 6-31G(d,p) has a higher level of accuracy than 3-21G. However, it takes longer time for calculation. Therefore, 3-21G was carried out first, to obtain a result close to the correct value, and then improve the accuracy of the result with 6-31G(d,p) basis set.
Set-up
- Method: B3LYP
- Basis set: 6-31G(d,p)
- Extra keywords: nosymm
- Job Type: Optimisation
- Keywords: # opt b3lyp/6-31g(d,p) geom=connectivity nosymm
Results
The log file of BH3 (opt 6-31G(d,p)) is available here.
- Summary Table
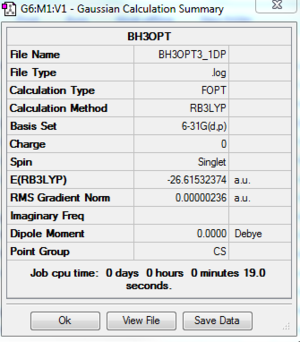
| File type | .log |
|---|---|
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Final Energy | -26.61532374 a.u. |
| Gradient | 0.000000236 a.u. |
| Dipole Moment | 0.0000 Debye |
| Point Group | CS |
| Calculation Time | 19.0 s |
- Item Table
Item Value Threshold Converged?
Maximum Force 0.000005 0.000450 YES
RMS Force 0.000003 0.000300 YES
Maximum Displacement 0.000020 0.001800 YES
RMS Displacement 0.000012 0.001200 YES
Predicted change in Energy=-1.312911D-10
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.1923 -DE/DX = 0.0 !
! R2 R(1,3) 1.1923 -DE/DX = 0.0 !
! R3 R(1,4) 1.1923 -DE/DX = 0.0 !
! A1 A(2,1,3) 120.0002 -DE/DX = 0.0 !
! A2 A(2,1,4) 120.0002 -DE/DX = 0.0 !
! A3 A(3,1,4) 119.9997 -DE/DX = 0.0 !
! D1 D(2,1,4,3) 180.0 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
Both the forces and the placements are converged.
- Other Results
B-H bond length = 1.19232 Å
H-B-H bond angle= 120
The length of B-H bonds and H-B-H bond angles are also all equal in this calculation. However, the bond length is lower than in the previous section. A lower bond length generally indicates a stronger bond and a lower energy. In this case, optimisation using 6-31G(d,p) basis set managed to significantly lower the final energy of the molecule, as shown in the next section.
Discussion
There was a change in the final energy after 6-31G(d,p) optimisation was carries out.
| Basis Set | Energy /a.u. |
|---|---|
| 3-21G | -26.46226338 |
| 6-31G(d,p) | -26.61532374 |
| Change in Energy | -0.15306036 |
The final energy obtained with 6-31G(d,p) basis set is 0.15306036 a.u. lower than using 3-21G basis set. This is equivalent to approximately -401.86 kJ/mol. This is a very large energy difference, considering it is higher than the enthalpy change of most of the common chemical reactions (eg. reaction between water and lithium only has an enthalpy change of approximately 200kJ/mol).
Optimisation of TlBr3 using 'B3LYP, 6-31G'
TlBr molecule |
TlBr3 is a much heavier molecule than BH3. The large number of electrons present in the molecule give rise to different properties as compared to BH3 molecule.
Set-up
- Method: B3LYP
- Basis set: 6-31G(d,p)
- Extra keywords: nosymm
- Job Type: Optimisation
- Keywords: # opt b3lyp/6-31g(d,p) geom=connectivity nosymm
Results
The log file of TlBr3 (opt 6-31G(d,p)) is available at [1].
- Summary Table
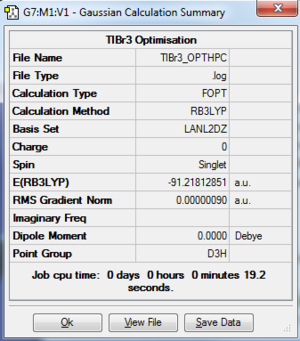
| File type | .log |
|---|---|
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Final Energy | -91.21812851 a.u. |
| Gradient | 0.00000090 a.u. |
| Dipole Moment | 0.0000 Debye |
| Point Group | D3h |
| Calculation Time | 19.2 s |
- Item Table
Item Value Threshold Converged?
Maximum Force 0.000002 0.000450 YES
RMS Force 0.000001 0.000300 YES
Maximum Displacement 0.000022 0.001800 YES
RMS Displacement 0.000014 0.001200 YES
Predicted change in Energy=-6.082962D-11
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 2.651 -DE/DX = 0.0 !
! R2 R(1,3) 2.651 -DE/DX = 0.0 !
! R3 R(1,4) 2.651 -DE/DX = 0.0 !
! A1 A(2,1,3) 120.0 -DE/DX = 0.0 !
! A2 A(2,1,4) 120.0 -DE/DX = 0.0 !
! A3 A(3,1,4) 120.0 -DE/DX = 0.0 !
! D1 D(2,1,4,3) 180.0 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
Both the forces and the placements are converged.
- Other Results
Tl-Br bond length = 2.65095 Å
Br-Tl-Br bond angle= 120
The length of Tl-Br bonds and Br-Tl-Br bond angles are also all equal in this calculation.
The literature values:
Optimisation of BBr3 using 'B3LYP, GEN'
BBr molecule |
Set-up
- Method: B3LYP
- Basis set: GEN
- Extra keywords: pseudo=cards gfinput
- Job Type: Optimisation
- Keywords: # opt b3lyp/gen nosymm geom=connectivity gfinput pseudo=cards
Results
The log file of BBr3 (opt B3LYP/GEN) is available here.
- Summary Table
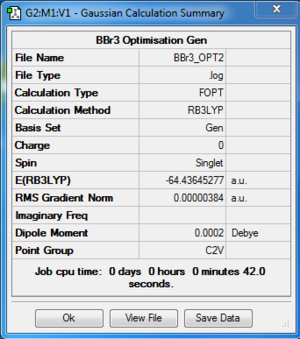
| File type | .log |
|---|---|
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | Gen |
| Final Energy | -64.43645277 a.u. |
| Gradient | 0.000000384 a.u. |
| Dipole Moment | 0.0002 Debye |
| Point Group | C2V |
| Calculation Time | 42.0 s |
- Item Table
Item Value Threshold Converged?
Maximum Force 0.000008 0.000450 YES
RMS Force 0.000005 0.000300 YES
Maximum Displacement 0.000036 0.001800 YES
RMS Displacement 0.000024 0.001200 YES
Predicted change in Energy=-4.098488D-10
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.9339 -DE/DX = 0.0 !
! R2 R(1,3) 1.934 -DE/DX = 0.0 !
! R3 R(1,4) 1.934 -DE/DX = 0.0 !
! A1 A(2,1,3) 120.0022 -DE/DX = 0.0 !
! A2 A(2,1,4) 120.0022 -DE/DX = 0.0 !
! A3 A(3,1,4) 119.9956 -DE/DX = 0.0 !
! D1 D(2,1,4,3) 180.0 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
Both the forces and the placements are converged.
- Other Results
B-Br bond length = 1.93394 Å, 1.93397 Å, 1.93397 Å
Br-B-Br bond angle= 119.996, 120.002, 120.002
There are small fluctuations in bond lengths and bond angles. However, the difference is trivial due to the limited accuracy of the calculation method. Therefore, this difference is ignored and the bond angle is taken as 120 and the bond length is taken as 1.9340 Å.
Discussion
The bond lengths and bond angles of BH3 , BBr3 and TlBr3 are compared and discussed in this section.
| Molecule | Bond Length /Å | Bond Angle |
|---|---|---|
| BH3 | 1.19349 | 120.0 |
| BBr3 | 1.9340 | 120.0 |
| TlBr3 | 2.65095 | 120.0 |
It is observed that the bond length increases down the table while the bond angle maintains the same. Although the atoms in the molecules changed, all of these 3 molecules have the same trigonal planar structure. This is because within each molecule, the 3 bonds are equivalent. The bond angle depends on the structure of the molecule. Therefore, all the molecules have a bond angle of 120 degree. In another words, as long as the structure remains the same, even if the central atom or the ligands are changed, the bond angle would still remain the same.
However, the bond length largely depends on the sizes of the individual atoms. Since TlBr3 has most number of electrons and the largest individual atoms sizes, followed by BBr3. The bond length of TlBr3>BBr3>BH3. It can also be observed that when the ligand is large, with a large number of electrons, the bond length would increase, as when H in BH3 is replaced by Br. And when the central atoms is replaced by a large atom, the bond length would also increase, as observed when B in BBr3 is replaced by Tl.
- Bonding in Gaussview
A bond represents the attractive interaction between 2 or more atoms. It may not be a simple force but a combined effect in some cases. For example, a bond strength can be affected by the repulsion of nuclei or the attraction between the oppositely charged ions. Therefore, the bond strength vary with distance. At a point when the interaction is considered too low, the bond will not be shown in Gaussview. However, it does not mean that there is no interaction between the atoms, just too weak to be considered as a significant bond.
Frequency Analysis, Population Analysis and Association Energy
This section contains the results obtained from the exercises in Day 3 and 4.
Frequency Analysis of BH3
After completing the optimisation of BH3 molecule using 3-21G basis set, the molecule was further optimised with 6-31G(d,p) basis set. 6-31G(d,p) has a higher level of accuracy than 3-21G. However, it takes longer time for calculation. Therefore, 3-21G was carried out first, to obtain a result close to the correct value, and then improve the accuracy of the result with 6-31G(d,p) basis set.
Set-up
- Method: B3LYP
- Basis set: 6-31G(d,p)
- Extra keywords: nosymm
- Job Type: Frequency
- Keywords: # freq b3lyp/6-31g(d,p) nosymm geom=connectivity
Results
The log file of BH3 (freq 6-31G(d,p)) is available here.
- Summary Table

| File type | .log |
|---|---|
| Calculation Type | FREQ |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Final Energy | -26.61532374 a.u. |
| Gradient | 0.000000237 a.u. |
| Dipole Moment | 0.0000 Debye |
| Point Group | C2V |
| Calculation Time | 10.0 s |
- Low Frequencies Table
Low frequencies --- -18.6669 -0.0009 -0.0003 0.0006 12.5167 12.5631 Low frequencies --- 1162.9785 1213.1756 1213.2363
The low frequencies
- Infra-red Spectrum
There are 3 peaks observed from the spectrum. However, there are 6 allowed vibrations as shown below. The reason for the 3 missing peaks are explained in the next section, under discussion.
- Vibrations
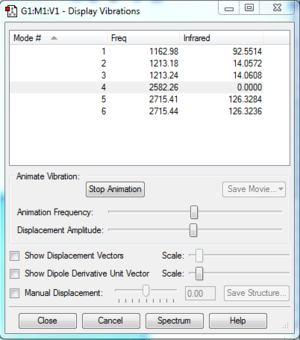
Discussion
- Missing Peaks from the IR Spectrum

There are 6 allowed vibrations, but only 3 visible peaks are observed from the spectrum. There are 2 reasons for the missing peaks.
1. Some vibrations are very close in frequency, and merged on the spectrum to show one single peak. For example, as shown on the spectrum on the right, the second peak on the spectrum is actually combined from vibration mode 1 (1213.18 cm-1) and 2 (1213.24 cm-1). Only when the spectrum is expanded, 2 lines are observed, indicating that they are different vibration modes. The same observation was made for vibration mode 5 and 6, which merged to form the third peak on the spectra.
2. Some vibration modes are allowed, but the intensity was too low to be observed. For example, the intensity of vibration mode 4 (2582.26 cm-1) is 0.
Frequency Analysis of TlBr3
Set-up
- Method: B3LYP
- Basis set: 6-31G(d,p)
- Extra keywords: nosymm
- Job Type: Frequency
- Keywords: # freq b3lyp/6-31g(d,p) nosymm geom=connectivity
Results
The log file of TlBr3 (freq 6-31G(d,p)) is available at [[2]].
- Summary Table
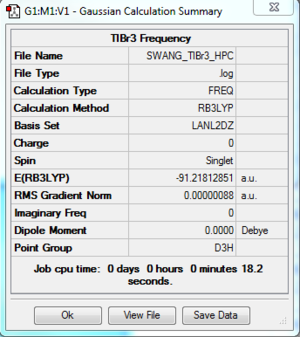
| File type | .log |
|---|---|
| Calculation Type | FREQ |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Final Energy | -91.21812851 a.u. |
| Gradient | 0.00000088 a.u. |
| Dipole Moment | 0.0000 Debye |
| Point Group | D3h |
| Calculation Time | 18.2 s |
- Low Frequencies Table
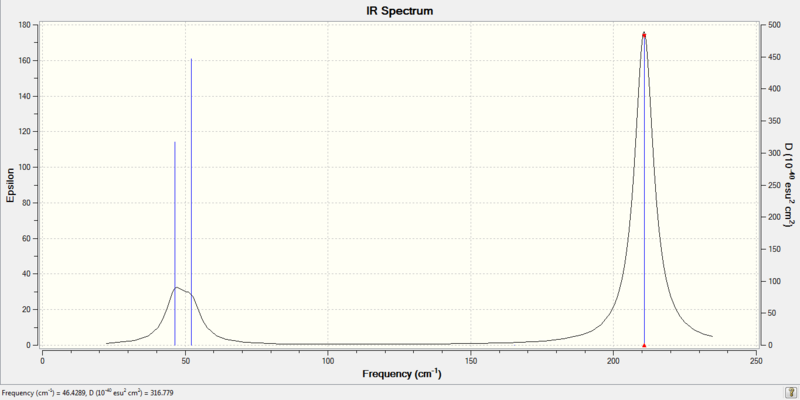
Low frequencies --- -3.4213 -0.0026 -0.0004 0.0015 3.9362 3.9362 Low frequencies --- 46.4289 46.4292 52.1449
- Lowest Real Normal Mode?
- Infra-red Spectrum
- Vibrations
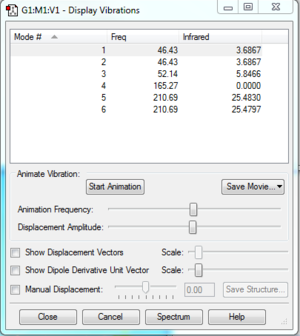
Discussion
- Infrared Sprectrum
Both spectra are very similar. Although the frequencies are very different, similar pattern was observed. Both spectra have 2 small peak with lower intensity on the left and a large peak on the right side. The frenquencies of BH3 is much lareger than TlBr3. This is because B and H atoms are much smaller and lighter than Tl and Br. Much less energy is required to move B and H around and they can move much faster when energy is applied. A higher frequency also shows that BH3 have higher vibrational energy.
Reordering of modes was also observed. The frequency is highly dependent on the mass of the atoms. However, the effect of atoms on each vibration mode maybe different. For example, certain atoms are stationary in certain motions and hence the mass of that atom have less effect on the frequency. In another words, the change in mass of the atom would cause different levels of change in the frequency, which leads to reordering.
- Frequency analysis
- Why must you use the same method and basis set for both the optimisation and frequency analysis calculations?
Different methods and basis sets would mean that the calculation would be done differently, with different level of accuracy and the error of the results would be different as well. The difference in energy can be as high as 400kJ/mol, as shown in section 1.1.2. The error may even be higher than the difference between the 2 values being compared, which makes the answer very inaccurate and meaningless. Hence, it is important to ensure that all the analysis are made based on same set-up, to ensure that the answers would have similar level of accuracy and the error would be similar, or in the same order of magnitude.
- What is the purpose of carrying out a frequency analysis?
A frequency analysis enables us to ensure that the optimisation was done correctly. For the optimisation to be successful, it has to be either a highest point/peak (transition state) or a lowest point/trough (ground state). This is carried out by taking the first and second derivatives. At any stationary point, the first derivative should be close to 0.
The frequency analysis test the second derivative. If all second derivative are positive, a minima(ground state) is obtained. If one negative value is obtained, then a maxima(transition state) is obtained. If more than 1 negative value is obtained, the optimisation is not successful.
Population Analysis of BH3
After completing the optimisation of BH3 molecule using 3-21G basis set, the molecule was further optimised with 6-31G(d,p) basis set. 6-31G(d,p) has a higher level of accuracy than 3-21G. However, it takes longer time for calculation. Therefore, 3-21G was carried out first, to obtain a result close to the correct value, and then improve the accuracy of the result with 6-31G(d,p) basis set.
Set-up
- Method: B3LYP
- Basis set: 6-31G(d,p)
- NBO: Full NBO
- Extra keywords: pop=full
- Job Type: Energy
- Keywords: # b3lyp/6-31g(d,p) pop=(nbo,full) geom=connectivity
Results
The log file of BH3 (pop 6-31G(d,p)) is available here and the link to check-point file on D-space is available [[Media:|here]].
- Summary Table
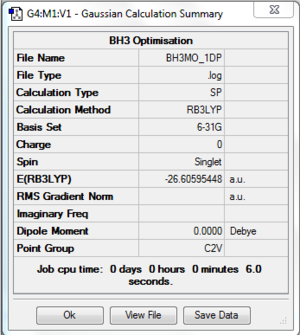
| File type | .log |
|---|---|
| Calculation Type | SP |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Final Energy | -26.60595448 a.u. |
| Gradient | a.u. |
| Dipole Moment | 0.0000 Debye |
| Point Group | C2V |
| Calculation Time | 6.0 s |
Discussion
- Molecular Orbitals of BH3
The theoretical molecular orbitals are
| Real MO |  a1' |
 e' |
 e' |
 a2'
|
|---|---|---|---|---|
| Theoretical MO |  |
 |
 |
 |
NBO Analysis of NH3
BH molecule |
Set-up
1. B3LYP/6_31G(d,p)
- Method: B3LYP
- Basis set: 6-31G(d,p)
- Extra keywords: nosymm
- Job Type: Optimisation
- Keywords: # opt b3lyp/6-31g(d,p) geom=connectivity nosymm
2. B3LYP/Frequency
- Method: B3LYP
- Basis set: 6-31G(d,p)
- Extra keywords: nosymm
- Job Type: Frequency
- Keywords: # freq b3lyp/6-31g(d,p) nosymm geom=connectivity
3. B3LYP/Population
- Method: B3LYP
- Basis set: 6-31G(d,p)
- NBO: Full NBO
- Extra keywords: pop=full
- Job Type: Energy
- Keywords: # b3lyp/6-31g(d,p) pop=(nbo,full) geom=connectivity
Results
- 1. B3LYP/6_31G(d,p) Optimisation
The log file for 6-31G(d,p) optimisation of NH3 is available here.
Summary Table
[[Image:|thumb|widthpx|]]
| File type | .log |
|---|---|
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Final Energy | -56.55776856 a.u. |
| Gradient | 0.00000885 a.u. |
| Dipole Moment | 1.8464 Debye |
| Point Group | C1 |
| Calculation Time | 25.0 s |
Item Table
Item Value Threshold Converged?
Maximum Force 0.000024 0.000450 YES
RMS Force 0.000012 0.000300 YES
Maximum Displacement 0.000079 0.001800 YES
RMS Displacement 0.000053 0.001200 YES
Predicted change in Energy=-1.629727D-09
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.018 -DE/DX = 0.0 !
! R2 R(1,3) 1.018 -DE/DX = 0.0 !
! R3 R(1,4) 1.018 -DE/DX = 0.0 !
! A1 A(2,1,3) 105.7413 -DE/DX = 0.0 !
! A2 A(2,1,4) 105.7486 -DE/DX = 0.0 !
! A3 A(3,1,4) 105.7479 -DE/DX = 0.0 !
! D1 D(2,1,4,3) -111.8631 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
- Both the forces and the placements are converged.
- 2. B3LYP/Frequency
The log file for frequency optimisation of NH3 is available here.
Summary Table
[[Image:|thumb|widthpx|]]
| File type | .log |
|---|---|
| Calculation Type | FREQ |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Final Energy | -56.55776856 a.u. |
| Gradient | 0.00000890 a.u. |
| Dipole Moment | 1.8464 Debye |
| Point Group | C1 |
| Calculation Time | 12.0 s |
Low Frequencies Table
Low frequencies --- -30.7812 0.0013 0.0014 0.0016 20.2807 28.2444 Low frequencies --- 1089.5535 1694.1234 1694.1866
- 3. B3LYP/Population
The log file for population analysis of NH3 is available here.
- Summary Table
[[Image:|thumb|widthpx| ]]
| File type | .log |
|---|---|
| Calculation Type | SP |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Final Energy | -56.55776856 a.u. |
| Gradient | a.u. |
| Dipole Moment | 1.8464 Debye |
| Point Group | C1 |
| Calculation Time | 9.0 s |
- Charge Distribution by Colour
The range was set between -1 and 1. 
- Charge Distribution by Numbers
Association Energy of NH3BH3
BH molecule |
In this section, the association energy of NH3BH3 would be identified by looking at the difference between its final energy, as compared to the sum of final energies from NH3 and BH3 individually. To ensure the result is accurate, the same optimisation process was carried out on NH3BH3. Due to the large number of atoms of NH3BH3 as compared to NH3, optimisation was first carried out 3-21G to reduce the calculation time (which is not necessary for small molecules like NH3).
Set-up
1. B3LYP/3-21G
- Method: B3LYP
- Basis set: 3-21G
- Job Type: Optimisation
- Keywords: # opt b3lyp/3-21g geom=connectivity
2. B3LYP/6_31G(d,p)
- Method: B3LYP
- Basis set: 6-31G(d,p)
- Extra keywords: nosymm
- Job Type: Optimisation
- Keywords: # opt b3lyp/6-31g(d,p) geom=connectivity nosymm
3. B3LYP/Frequency
- Method: B3LYP
- Basis set: 6-31G(d,p)
- Extra keywords: nosymm
- Job Type: Frequency
- Keywords: # freq b3lyp/6-31g(d,p) nosymm geom=connectivity
Results
- 1. B3LYP/3_21G Optimisation
- The log file for 3-21G optimisation of NH3BH3 is available here.
- Summary Table
[[|thumb|widthpx| ]]
| File type | .log |
|---|---|
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 3-21G |
| Final Energy | -82.76661837 a.u. |
| Gradient | 0.00003006 a.u. |
| Dipole Moment | 5.8431 Debye |
| Point Group | C1 |
| Calculation Time | 40.0 s |
- Item Table
Item Value Threshold Converged?
Maximum Force 0.000094 0.000450 YES
RMS Force 0.000030 0.000300 YES
Maximum Displacement 0.000419 0.001800 YES
RMS Displacement 0.000178 0.001200 YES
Predicted change in Energy=-5.742851D-08
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,7) 1.0277 -DE/DX = -0.0001 !
! R2 R(2,7) 1.0277 -DE/DX = -0.0001 !
! R3 R(3,7) 1.0277 -DE/DX = 0.0 !
! R4 R(4,8) 1.212 -DE/DX = 0.0 !
! R5 R(5,8) 1.212 -DE/DX = -0.0001 !
! R6 R(6,8) 1.212 -DE/DX = -0.0001 !
! R7 R(7,8) 1.6854 -DE/DX = -0.0001 !
! A1 A(1,7,2) 109.3469 -DE/DX = 0.0 !
! A2 A(1,7,3) 109.35 -DE/DX = 0.0 !
! A3 A(1,7,8) 109.5925 -DE/DX = 0.0 !
! A4 A(2,7,3) 109.3462 -DE/DX = 0.0 !
! A5 A(2,7,8) 109.5973 -DE/DX = 0.0 !
! A6 A(3,7,8) 109.5935 -DE/DX = 0.0 !
! A7 A(4,8,5) 113.563 -DE/DX = 0.0 !
! A8 A(4,8,6) 113.5594 -DE/DX = 0.0 !
! A9 A(4,8,7) 104.9872 -DE/DX = 0.0 !
! A10 A(5,8,6) 113.5575 -DE/DX = 0.0 !
! A11 A(5,8,7) 104.9846 -DE/DX = 0.0 !
! A12 A(6,8,7) 104.9848 -DE/DX = 0.0 !
! D1 D(1,7,8,4) 179.9938 -DE/DX = 0.0 !
! D2 D(1,7,8,5) -60.0024 -DE/DX = 0.0 !
! D3 D(1,7,8,6) 59.994 -DE/DX = 0.0 !
! D4 D(2,7,8,4) -60.0065 -DE/DX = 0.0 !
! D5 D(2,7,8,5) 59.9972 -DE/DX = 0.0 !
! D6 D(2,7,8,6) 179.9936 -DE/DX = 0.0 !
! D7 D(3,7,8,4) 59.9928 -DE/DX = 0.0 !
! D8 D(3,7,8,5) 179.9966 -DE/DX = 0.0 !
! D9 D(3,7,8,6) -60.007 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
Both the forces and the placements are converged.
- 2. B3LYP/6_31G(d,p) Optimisation
- The log file for 6-31G(d,p) optimisation of NH3BH3 is available here.
Summary Table
[[Image:|thumb|widthpx|]]
| File type | .log |
|---|---|
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Final Energy | -83.22469035 a.u. |
| Gradient | 0.00005813 a.u. |
| Dipole Moment | 5.5628 Debye |
| Point Group | C1 |
| Calculation Time | 38.0 s |
Item Table
Item Value Threshold Converged?
Maximum Force 0.000137 0.000450 YES
RMS Force 0.000038 0.000300 YES
Maximum Displacement 0.001020 0.001800 YES
RMS Displacement 0.000225 0.001200 YES
Predicted change in Energy=-1.139066D-07
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,7) 1.0185 -DE/DX = 0.0 !
! R2 R(2,7) 1.0185 -DE/DX = 0.0 !
! R3 R(3,7) 1.0185 -DE/DX = 0.0 !
! R4 R(4,8) 1.2097 -DE/DX = 0.0 !
! R5 R(5,8) 1.2097 -DE/DX = 0.0 !
! R6 R(6,8) 1.2097 -DE/DX = 0.0 !
! R7 R(7,8) 1.6685 -DE/DX = -0.0001 !
! A1 A(1,7,2) 107.8567 -DE/DX = 0.0 !
! A2 A(1,7,3) 107.8615 -DE/DX = 0.0 !
! A3 A(1,7,8) 111.0394 -DE/DX = 0.0 !
! A4 A(2,7,3) 107.861 -DE/DX = 0.0 !
! A5 A(2,7,8) 111.0396 -DE/DX = 0.0 !
! A6 A(3,7,8) 111.0358 -DE/DX = 0.0 !
! A7 A(4,8,5) 113.9015 -DE/DX = 0.0 !
! A8 A(4,8,6) 113.8953 -DE/DX = 0.0 !
! A9 A(4,8,7) 104.567 -DE/DX = 0.0001 !
! A10 A(5,8,6) 113.9013 -DE/DX = 0.0 !
! A11 A(5,8,7) 104.5643 -DE/DX = 0.0001 !
! A12 A(6,8,7) 104.5652 -DE/DX = 0.0001 !
! D1 D(1,7,8,4) -179.9977 -DE/DX = 0.0 !
! D2 D(1,7,8,5) -59.9951 -DE/DX = 0.0 !
! D3 D(1,7,8,6) 60.0064 -DE/DX = 0.0 !
! D4 D(2,7,8,4) -59.9999 -DE/DX = 0.0 !
! D5 D(2,7,8,5) 60.0027 -DE/DX = 0.0 !
! D6 D(2,7,8,6) -179.9958 -DE/DX = 0.0 !
! D7 D(3,7,8,4) 60.001 -DE/DX = 0.0 !
! D8 D(3,7,8,5) -179.9965 -DE/DX = 0.0 !
! D9 D(3,7,8,6) -59.995 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
- Both the forces and the placements are converged.
- 3. B3LYP/Frequency
The log file for frequency optimisation of NH3BH3 is available here.
Summary Table
[[Image:|thumb|widthpx|]]
| File type | .log |
|---|---|
| Calculation Type | FREQ |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Final Energy | -83.22469035 a.u. |
| Gradient | 0.00005811 a.u. |
| Dipole Moment | 5.5628 Debye |
| Point Group | C1 |
| Calculation Time | 46.0 s |
Low Frequencies Table
Low frequencies --- -0.0010 0.0011 0.0012 9.3469 10.9471 30.1237 Low frequencies --- 264.8041 631.3745 637.2816
Discussion
- Association Energy (A.E)
=E(NH3BH3)-E(NH3)-E(BH3)
=-83.22469035+56.55776856+26.61532374
=-0.05159805 a.u.
=-135.47 kJ/mol
Week 2 Project: Optimisation and Analysis of Aromatic Molecules
In this section, a mini project on aromaticity is carried out. 4 molecules are studied in this section: Benzene, pyradinium, boratabenzene and borazine. They are optimised to the same level. The effect of the structures on their molecular orbitals and other properties are also discussed.
Optimisation
Benzene
TlBr molecule |
- 1. B3LYP/6_31G(d,p) Optimisation
- Method: B3LYP
- Basis set: 6-31G(d,p)
- Extra keywords: nosymm
- Job Type: Optimisation
- Keywords: # opt b3lyp/6-31g(d,p) geom=connectivity nosymm
- The log file for 6-31G(d,p) optimisation of Benzene is available at [[3]].
Summary Table
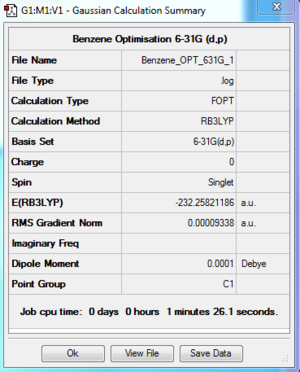
| File type | .log |
|---|---|
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Final Energy | -232.25821186 a.u. |
| Gradient | 0.00009338 a.u. |
| Dipole Moment | 0.0001 Debye |
| Point Group | C1 |
| Calculation Time | 1 min 26.1 s |
Item Table
Item Value Threshold Converged?
Maximum Force 0.000204 0.000450 YES
RMS Force 0.000084 0.000300 YES
Maximum Displacement 0.000870 0.001800 YES
RMS Displacement 0.000313 0.001200 YES
Predicted change in Energy=-4.983462D-07
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.3963 -DE/DX = 0.0001 !
! R2 R(1,6) 1.3961 -DE/DX = 0.0002 !
! R3 R(1,7) 1.0861 -DE/DX = 0.0002 !
! R4 R(2,3) 1.3961 -DE/DX = 0.0002 !
! R5 R(2,8) 1.0861 -DE/DX = 0.0002 !
! R6 R(3,4) 1.3963 -DE/DX = 0.0001 !
! R7 R(3,9) 1.0861 -DE/DX = 0.0002 !
! R8 R(4,5) 1.3961 -DE/DX = 0.0002 !
! R9 R(4,10) 1.0861 -DE/DX = 0.0002 !
! R10 R(5,6) 1.3963 -DE/DX = 0.0001 !
! R11 R(5,11) 1.0861 -DE/DX = 0.0002 !
! R12 R(6,12) 1.0861 -DE/DX = 0.0002 !
! A1 A(2,1,6) 119.9996 -DE/DX = 0.0 !
! A2 A(2,1,7) 119.9968 -DE/DX = 0.0 !
! A3 A(6,1,7) 120.0036 -DE/DX = 0.0 !
! A4 A(1,2,3) 120.0036 -DE/DX = 0.0 !
! A5 A(1,2,8) 119.9916 -DE/DX = 0.0 !
! A6 A(3,2,8) 120.0048 -DE/DX = 0.0 !
! A7 A(2,3,4) 119.9967 -DE/DX = 0.0 !
! A8 A(2,3,9) 120.0101 -DE/DX = 0.0 !
! A9 A(4,3,9) 119.9932 -DE/DX = 0.0 !
! A10 A(3,4,5) 119.9996 -DE/DX = 0.0 !
! A11 A(3,4,10) 119.9891 -DE/DX = 0.0 !
! A12 A(5,4,10) 120.0113 -DE/DX = 0.0 !
! A13 A(4,5,6) 120.004 -DE/DX = 0.0 !
! A14 A(4,5,11) 120.0048 -DE/DX = 0.0 !
! A15 A(6,5,11) 119.9912 -DE/DX = 0.0 !
! A16 A(1,6,5) 119.9965 -DE/DX = 0.0 !
! A17 A(1,6,12) 120.0072 -DE/DX = 0.0 !
! A18 A(5,6,12) 119.9963 -DE/DX = 0.0 !
! D1 D(6,1,2,3) -0.0059 -DE/DX = 0.0 !
! D2 D(6,1,2,8) 180.0021 -DE/DX = 0.0 !
! D3 D(7,1,2,3) -180.0099 -DE/DX = 0.0 !
! D4 D(7,1,2,8) -0.0019 -DE/DX = 0.0 !
! D5 D(2,1,6,5) -0.0055 -DE/DX = 0.0 !
! D6 D(2,1,6,12) -179.9972 -DE/DX = 0.0 !
! D7 D(7,1,6,5) -180.0016 -DE/DX = 0.0 !
! D8 D(7,1,6,12) 0.0068 -DE/DX = 0.0 !
! D9 D(1,2,3,4) 0.0119 -DE/DX = 0.0 !
! D10 D(1,2,3,9) 180.0087 -DE/DX = 0.0 !
! D11 D(8,2,3,4) 180.0039 -DE/DX = 0.0 !
! D12 D(8,2,3,9) 0.0007 -DE/DX = 0.0 !
! D13 D(2,3,4,5) -0.0064 -DE/DX = 0.0 !
! D14 D(2,3,4,10) -180.0058 -DE/DX = 0.0 !
! D15 D(9,3,4,5) 179.9968 -DE/DX = 0.0 !
! D16 D(9,3,4,10) -0.0026 -DE/DX = 0.0 !
! D17 D(3,4,5,6) -0.005 -DE/DX = 0.0 !
! D18 D(3,4,5,11) 180.0061 -DE/DX = 0.0 !
! D19 D(10,4,5,6) -180.0057 -DE/DX = 0.0 !
! D20 D(10,4,5,11) 0.0055 -DE/DX = 0.0 !
! D21 D(4,5,6,1) 0.011 -DE/DX = 0.0 !
! D22 D(4,5,6,12) 180.0027 -DE/DX = 0.0 !
! D23 D(11,5,6,1) 179.9999 -DE/DX = 0.0 !
! D24 D(11,5,6,12) -0.0085 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
- Both the forces and the placements are converged.
- 2. B3LYP/Frequency
- Method: B3LYP
- Basis set: 6-31G(d,p)
- Extra keywords: nosymm
- Job Type: Frequency
- Keywords: # freq b3lyp/6-31g(d,p) nosymm geom=connectivity
- The log file for frequency optimisation of Benzene is available at
[[4]].
Summary Table

| File type | .log |
|---|---|
| Calculation Type | FREQ |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Final Energy | -232.25821186 a.u. |
| Gradient | 0.00009340 a.u. |
| Dipole Moment | 0.0001 Debye |
| Point Group | C1 |
| Calculation Time | 4 min 50.6 s |
Low Frequencies Table
Low frequencies --- -14.2239 -2.7052 0.0003 0.0008 0.0009 10.0128 Low frequencies --- 413.7276 414.5534 621.0441
- 3. B3LYP/Population
- Method: B3LYP
- Basis set: 6-31G(d,p)
- NBO: Full NBO
- Extra keywords: pop=full
- Job Type: Energy
- Keywords: # b3lyp/6-31g(d,p) pop=(nbo,full) geom=connectivity
- The log file for population analysis of Benzene is available [[5]].
- Summary Table

| File type | .log |
|---|---|
| Calculation Type | SP |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Final Energy | -26.60595448 a.u. |
| Gradient | a.u. |
| Dipole Moment | 0.0000 Debye |
| Point Group | C2V |
| Calculation Time | 6.0 s |
Pyridinium
TlBr molecule |
- 1. B3LYP/6_31G(d,p) Optimisation
- Method: B3LYP
- Basis set: 6-31G(d,p)
- Extra keywords: nosymm
- Job Type: Optimisation
- Keywords: # opt b3lyp/6-31g(d,p) geom=connectivity nosymm
- The log file for 6-31G(d,p) optimisation of Pyridinium is available at [[6]].
Summary Table

| File type | .log |
|---|---|
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Final Energy | -248.66807395 a.u. |
| Gradient | 0.00003913 a.u. |
| Dipole Moment | 9.0687 Debye |
| Point Group | C1 |
| Calculation Time | 3 min 9.5 s |
Item Table
Item Value Threshold Converged?
Maximum Force 0.000064 0.000450 YES
RMS Force 0.000023 0.000300 YES
Maximum Displacement 0.000702 0.001800 YES
RMS Displacement 0.000176 0.001200 YES
Predicted change in Energy=-6.968238D-08
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.3839 -DE/DX = 0.0 !
! R2 R(1,5) 1.3988 -DE/DX = 0.0 !
! R3 R(1,6) 1.0835 -DE/DX = 0.0 !
! R4 R(2,7) 1.0832 -DE/DX = 0.0 !
! R5 R(2,12) 1.3523 -DE/DX = 0.0001 !
! R6 R(3,4) 1.3838 -DE/DX = 0.0 !
! R7 R(3,9) 1.0832 -DE/DX = 0.0 !
! R8 R(3,12) 1.3524 -DE/DX = 0.0 !
! R9 R(4,5) 1.3988 -DE/DX = 0.0 !
! R10 R(4,10) 1.0835 -DE/DX = 0.0 !
! R11 R(5,11) 1.0852 -DE/DX = 0.0 !
! R12 R(8,12) 1.0169 -DE/DX = 0.0 !
! A1 A(2,1,5) 119.082 -DE/DX = 0.0 !
! A2 A(2,1,6) 119.4192 -DE/DX = 0.0001 !
! A3 A(5,1,6) 121.4987 -DE/DX = -0.0001 !
! A4 A(1,2,7) 123.9294 -DE/DX = 0.0 !
! A5 A(1,2,12) 119.2362 -DE/DX = 0.0 !
! A6 A(7,2,12) 116.8344 -DE/DX = 0.0 !
! A7 A(4,3,9) 123.9326 -DE/DX = 0.0 !
! A8 A(4,3,12) 119.2355 -DE/DX = 0.0 !
! A9 A(9,3,12) 116.832 -DE/DX = 0.0 !
! A10 A(3,4,5) 119.0826 -DE/DX = 0.0 !
! A11 A(3,4,10) 119.4215 -DE/DX = 0.0 !
! A12 A(5,4,10) 121.4958 -DE/DX = -0.0001 !
! A13 A(1,5,4) 120.0549 -DE/DX = 0.0 !
! A14 A(1,5,11) 119.974 -DE/DX = 0.0 !
! A15 A(4,5,11) 119.9711 -DE/DX = 0.0 !
! A16 A(2,12,3) 123.3087 -DE/DX = 0.0 !
! A17 A(2,12,8) 118.3464 -DE/DX = 0.0 !
! A18 A(3,12,8) 118.3448 -DE/DX = 0.0 !
! D1 D(5,1,2,7) -180.0014 -DE/DX = 0.0 !
! D2 D(5,1,2,12) 0.0031 -DE/DX = 0.0 !
! D3 D(6,1,2,7) -0.0012 -DE/DX = 0.0 !
! D4 D(6,1,2,12) 180.0032 -DE/DX = 0.0 !
! D5 D(2,1,5,4) -0.0003 -DE/DX = 0.0 !
! D6 D(2,1,5,11) -180.0014 -DE/DX = 0.0 !
! D7 D(6,1,5,4) -180.0005 -DE/DX = 0.0 !
! D8 D(6,1,5,11) -0.0015 -DE/DX = 0.0 !
! D9 D(1,2,12,3) -0.0045 -DE/DX = 0.0 !
! D10 D(1,2,12,8) -180.0031 -DE/DX = 0.0 !
! D11 D(7,2,12,3) -180.0003 -DE/DX = 0.0 !
! D12 D(7,2,12,8) 0.001 -DE/DX = 0.0 !
! D13 D(9,3,4,5) 180.0008 -DE/DX = 0.0 !
! D14 D(9,3,4,10) -0.0003 -DE/DX = 0.0 !
! D15 D(12,3,4,5) 0.0 -DE/DX = 0.0 !
! D16 D(12,3,4,10) -180.001 -DE/DX = 0.0 !
! D17 D(4,3,12,2) 0.0029 -DE/DX = 0.0 !
! D18 D(4,3,12,8) 180.0015 -DE/DX = 0.0 !
! D19 D(9,3,12,2) 180.0022 -DE/DX = 0.0 !
! D20 D(9,3,12,8) 0.0009 -DE/DX = 0.0 !
! D21 D(3,4,5,1) -0.0012 -DE/DX = 0.0 !
! D22 D(3,4,5,11) -180.0002 -DE/DX = 0.0 !
! D23 D(10,4,5,1) 179.9999 -DE/DX = 0.0 !
! D24 D(10,4,5,11) 0.0009 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
- Both the forces and the placements are converged.
- 2. B3LYP/Frequency
- Method: B3LYP
- Basis set: 6-31G(d,p)
- Extra keywords: nosymm
- Job Type: Frequency
- Keywords: # freq b3lyp/6-31g(d,p) nosymm geom=connectivity
- The log file for frequency optimisation of Pyridinium is available at [[7]].
Summary Table
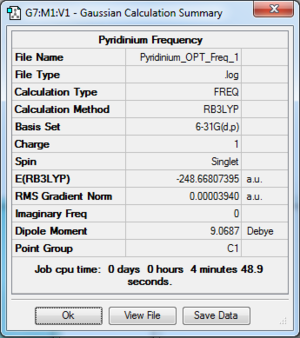
| File type | .log |
|---|---|
| Calculation Type | FREQ |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Final Energy | -248.66807395 a.u. |
| Gradient | 0.00003940 a.u. |
| Dipole Moment | 9.0687 Debye |
| Point Group | C1 |
| Calculation Time | 4 min 48.9 s |
Low Frequencies Table
Low frequencies --- -7.2037 -0.0010 -0.0008 -0.0007 17.3393 18.5369 Low frequencies --- 392.4558 404.0618 620.4715
- 3. B3LYP/Population
- Method: B3LYP
- Basis set: 6-31G(d,p)
- NBO: Full NBO
- Extra keywords: pop=full
- Job Type: Energy
- Keywords: # b3lyp/6-31g(d,p) pop=(nbo,full) geom=connectivity
- The log file for population analysis of Pyridinium is available at [[8]].
- Summary Table

| File type | .log |
|---|---|
| Calculation Type | SP |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Final Energy | -26.60595448 a.u. |
| Gradient | a.u. |
| Dipole Moment | 0.0000 Debye |
| Point Group | C2V |
| Calculation Time | 6.0 s |
Boratabenzene
TlBr molecule |
- 1. B3LYP/6_31G(d,p) Optimisation
- Method: B3LYP
- Basis set: 6-31G(d,p)
- Extra keywords: nosymm
- Job Type: Optimisation
- Keywords: # opt b3lyp/6-31g(d,p) geom=connectivity nosymm
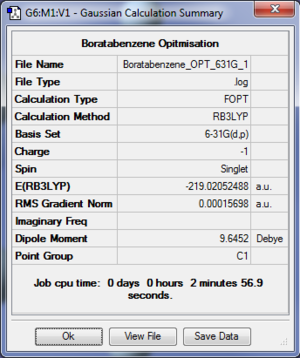
- The log file for 6-31G(d,p) optimisation of Boratabenzene is available at [[9]].
Summary Table
| File type | .log |
|---|---|
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Final Energy | -219.02052488 a.u. |
| Gradient | 0.00015698 a.u. |
| Dipole Moment | 9.6452 Debye |
| Point Group | C1 |
| Calculation Time | 2 min 56.9 s |
Item Table
Item Value Threshold Converged?
Maximum Force 0.000159 0.000450 YES
RMS Force 0.000068 0.000300 YES
Maximum Displacement 0.000904 0.001800 YES
RMS Displacement 0.000328 0.001200 YES
Predicted change in Energy=-6.532939D-07
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.4053 -DE/DX = -0.0001 !
! R2 R(1,5) 1.4053 -DE/DX = -0.0001 !
! R3 R(1,6) 1.0917 -DE/DX = -0.0001 !
! R4 R(2,3) 1.3989 -DE/DX = 0.0 !
! R5 R(2,7) 1.0968 -DE/DX = 0.0001 !
! R6 R(3,8) 1.097 -DE/DX = -0.0001 !
! R7 R(3,12) 1.5138 -DE/DX = 0.0001 !
! R8 R(4,5) 1.399 -DE/DX = 0.0 !
! R9 R(4,10) 1.097 -DE/DX = -0.0001 !
! R10 R(4,12) 1.5137 -DE/DX = 0.0001 !
! R11 R(5,11) 1.0968 -DE/DX = 0.0001 !
! R12 R(9,12) 1.2185 -DE/DX = 0.0 !
! A1 A(2,1,5) 120.4537 -DE/DX = -0.0001 !
! A2 A(2,1,6) 119.7691 -DE/DX = 0.0001 !
! A3 A(5,1,6) 119.7772 -DE/DX = 0.0001 !
! A4 A(1,2,3) 122.137 -DE/DX = 0.0001 !
! A5 A(1,2,7) 117.4404 -DE/DX = 0.0 !
! A6 A(3,2,7) 120.4226 -DE/DX = -0.0002 !
! A7 A(2,3,8) 115.9589 -DE/DX = 0.0001 !
! A8 A(2,3,12) 120.0799 -DE/DX = -0.0001 !
! A9 A(8,3,12) 123.9612 -DE/DX = -0.0001 !
! A10 A(5,4,10) 115.9457 -DE/DX = 0.0001 !
! A11 A(5,4,12) 120.0802 -DE/DX = -0.0001 !
! A12 A(10,4,12) 123.9741 -DE/DX = -0.0001 !
! A13 A(1,5,4) 122.1359 -DE/DX = 0.0001 !
! A14 A(1,5,11) 117.4344 -DE/DX = 0.0 !
! A15 A(4,5,11) 120.4297 -DE/DX = -0.0002 !
! A16 A(3,12,4) 115.1133 -DE/DX = 0.0 !
! A17 A(3,12,9) 122.4423 -DE/DX = 0.0 !
! A18 A(4,12,9) 122.4444 -DE/DX = 0.0 !
! D1 D(5,1,2,3) 0.0064 -DE/DX = 0.0 !
! D2 D(5,1,2,7) 179.9963 -DE/DX = 0.0 !
! D3 D(6,1,2,3) 180.0063 -DE/DX = 0.0 !
! D4 D(6,1,2,7) -0.0039 -DE/DX = 0.0 !
! D5 D(2,1,5,4) 0.0009 -DE/DX = 0.0 !
! D6 D(2,1,5,11) -180.0039 -DE/DX = 0.0 !
! D7 D(6,1,5,4) 180.0011 -DE/DX = 0.0 !
! D8 D(6,1,5,11) -0.0037 -DE/DX = 0.0 !
! D9 D(1,2,3,8) -180.0066 -DE/DX = 0.0 !
! D10 D(1,2,3,12) -0.0084 -DE/DX = 0.0 !
! D11 D(7,2,3,8) 0.0038 -DE/DX = 0.0 !
! D12 D(7,2,3,12) 180.002 -DE/DX = 0.0 !
! D13 D(2,3,12,4) 0.0034 -DE/DX = 0.0 !
! D14 D(2,3,12,9) 180.0026 -DE/DX = 0.0 !
! D15 D(8,3,12,4) 180.0015 -DE/DX = 0.0 !
! D16 D(8,3,12,9) 0.0007 -DE/DX = 0.0 !
! D17 D(10,4,5,1) 179.9967 -DE/DX = 0.0 !
! D18 D(10,4,5,11) 0.0016 -DE/DX = 0.0 !
! D19 D(12,4,5,1) -0.0057 -DE/DX = 0.0 !
! D20 D(12,4,5,11) -180.0008 -DE/DX = 0.0 !
! D21 D(5,4,12,3) 0.0034 -DE/DX = 0.0 !
! D22 D(5,4,12,9) 180.0042 -DE/DX = 0.0 !
! D23 D(10,4,12,3) -179.9991 -DE/DX = 0.0 !
! D24 D(10,4,12,9) 0.0016 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
- Both the forces and the placements are converged.
- 2. B3LYP/Frequency
- Method: B3LYP
- Basis set: 6-31G(d,p)
- Extra keywords: nosymm
- Job Type: Frequency
- Keywords: # freq b3lyp/6-31g(d,p) nosymm geom=connectivity
- The log file for frequency optimisation of Boratabenzene is available at [[ http://hdl.handle.net/10042/20969]].
Summary Table
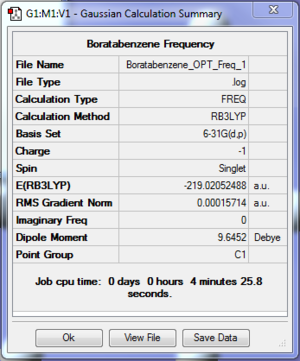
| File type | .log |
|---|---|
| Calculation Type | FREQ |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Final Energy | -219.02052488 a.u. |
| Gradient | 0.00015714 a.u. |
| Dipole Moment | 9.6452 Debye |
| Point Group | C1 |
| Calculation Time | 4 min 25.8 s |
Low Frequencies Table
Low frequencies --- -14.0457 -0.0009 -0.0008 -0.0003 9.6274 14.7083 Low frequencies --- 371.0134 404.6533 565.1739
- 3. B3LYP/Population
- Method: B3LYP
- Basis set: 6-31G(d,p)
- NBO: Full NBO
- Extra keywords: pop=full
- Job Type: Energy
- Keywords: # b3lyp/6-31g(d,p) pop=(nbo,full) geom=connectivity
- The log file for population analysis of Boratabenzene is available at [[10]].
- Summary Table

| File type | .log |
|---|---|
| Calculation Type | SP |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Final Energy | -26.60595448 a.u. |
| Gradient | a.u. |
| Dipole Moment | 0.0000 Debye |
| Point Group | C2V |
| Calculation Time | 6.0 s |
Borazine
TlBr molecule |
- 1. B3LYP/6_31G(d,p) Optimisation
- Method: B3LYP
- Basis set: 6-31G(d,p)
- Extra keywords: nosymm
- Job Type: Optimisation
- Keywords: # opt b3lyp/6-31g(d,p) geom=connectivity nosymm
- The log file for 6-31G(d,p) optimisation of Borazine is available at [[11]].
Summary Table
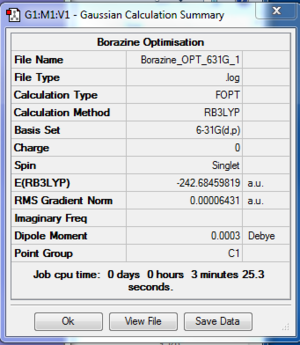
| File type | .log |
|---|---|
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Final Energy | -242.68459819 a.u. |
| Gradient | 0.00006431 a.u. |
| Dipole Moment | 0.0003 Debye |
| Point Group | C1 |
| Calculation Time | 3 min 25.3 s |
Item Table
Item Value Threshold Converged?
Maximum Force 0.000086 0.000450 YES
RMS Force 0.000033 0.000300 YES
Maximum Displacement 0.000253 0.001800 YES
RMS Displacement 0.000076 0.001200 YES
Predicted change in Energy=-9.359384D-08
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,12) 1.0097 -DE/DX = 0.0 !
! R2 R(2,8) 1.1949 -DE/DX = 0.0001 !
! R3 R(3,10) 1.0097 -DE/DX = 0.0 !
! R4 R(4,7) 1.1949 -DE/DX = 0.0001 !
! R5 R(5,11) 1.0097 -DE/DX = 0.0 !
! R6 R(6,9) 1.1949 -DE/DX = 0.0001 !
! R7 R(7,10) 1.4307 -DE/DX = -0.0001 !
! R8 R(7,11) 1.4307 -DE/DX = -0.0001 !
! R9 R(8,10) 1.4306 -DE/DX = -0.0001 !
! R10 R(8,12) 1.4307 -DE/DX = -0.0001 !
! R11 R(9,11) 1.4307 -DE/DX = -0.0001 !
! R12 R(9,12) 1.4307 -DE/DX = -0.0001 !
! A1 A(4,7,10) 121.4369 -DE/DX = 0.0 !
! A2 A(4,7,11) 121.4403 -DE/DX = 0.0 !
! A3 A(10,7,11) 117.1228 -DE/DX = 0.0 !
! A4 A(2,8,10) 121.4408 -DE/DX = 0.0 !
! A5 A(2,8,12) 121.4362 -DE/DX = 0.0 !
! A6 A(10,8,12) 117.123 -DE/DX = 0.0 !
! A7 A(6,9,11) 121.4376 -DE/DX = 0.0 !
! A8 A(6,9,12) 121.4413 -DE/DX = 0.0 !
! A9 A(11,9,12) 117.121 -DE/DX = 0.0 !
! A10 A(3,10,7) 118.56 -DE/DX = 0.0 !
! A11 A(3,10,8) 118.563 -DE/DX = 0.0 !
! A12 A(7,10,8) 122.877 -DE/DX = 0.0 !
! A13 A(5,11,7) 118.5638 -DE/DX = 0.0 !
! A14 A(5,11,9) 118.558 -DE/DX = 0.0 !
! A15 A(7,11,9) 122.8782 -DE/DX = 0.0 !
! A16 A(1,12,8) 118.5612 -DE/DX = 0.0 !
! A17 A(1,12,9) 118.5608 -DE/DX = 0.0 !
! A18 A(8,12,9) 122.878 -DE/DX = 0.0 !
! D1 D(4,7,10,3) -0.0008 -DE/DX = 0.0 !
! D2 D(4,7,10,8) -179.9997 -DE/DX = 0.0 !
! D3 D(11,7,10,3) 180.0 -DE/DX = 0.0 !
! D4 D(11,7,10,8) 0.0011 -DE/DX = 0.0 !
! D5 D(4,7,11,5) 0.0008 -DE/DX = 0.0 !
! D6 D(4,7,11,9) -179.998 -DE/DX = 0.0 !
! D7 D(10,7,11,5) -180.0 -DE/DX = 0.0 !
! D8 D(10,7,11,9) 0.0012 -DE/DX = 0.0 !
! D9 D(2,8,10,3) 0.0012 -DE/DX = 0.0 !
! D10 D(2,8,10,7) 180.0001 -DE/DX = 0.0 !
! D11 D(12,8,10,3) -180.001 -DE/DX = 0.0 !
! D12 D(12,8,10,7) -0.0021 -DE/DX = 0.0 !
! D13 D(2,8,12,1) -0.001 -DE/DX = 0.0 !
! D14 D(2,8,12,9) -180.0012 -DE/DX = 0.0 !
! D15 D(10,8,12,1) 180.0012 -DE/DX = 0.0 !
! D16 D(10,8,12,9) 0.0009 -DE/DX = 0.0 !
! D17 D(6,9,11,5) -0.0005 -DE/DX = 0.0 !
! D18 D(6,9,11,7) 179.9983 -DE/DX = 0.0 !
! D19 D(12,9,11,5) 179.9989 -DE/DX = 0.0 !
! D20 D(12,9,11,7) -0.0023 -DE/DX = 0.0 !
! D21 D(6,9,12,1) 0.0003 -DE/DX = 0.0 !
! D22 D(6,9,12,8) -179.9994 -DE/DX = 0.0 !
! D23 D(11,9,12,1) -179.9991 -DE/DX = 0.0 !
! D24 D(11,9,12,8) 0.0011 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
- Both the forces and the placements are converged.
- 2. B3LYP/Frequency
- Method: B3LYP
- Basis set: 6-31G(d,p)
- Extra keywords: nosymm
- Job Type: Frequency
- Keywords: # freq b3lyp/6-31g(d,p) nosymm geom=connectivity
- The log file for frequency optimisation of Borazine is available at [[12]].
Summary Table

| File type | .log |
|---|---|
| Calculation Type | FREQ |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Final Energy | -242.68459819 a.u. |
| Gradient | 0.00006441 a.u. |
| Dipole Moment | 0.0003 Debye |
| Point Group | C1 |
| Calculation Time | 4 min 23.5 s |
Low Frequencies Table
Low frequencies --- -7.1128 -0.0008 0.0007 0.0009 7.3502 13.0417 Low frequencies --- 288.5917 290.5607 404.2299
- 3. B3LYP/Population
- Method: B3LYP
- Basis set: 6-31G(d,p)
- NBO: Full NBO
- Extra keywords: pop=full
- Job Type: Energy
- Keywords: # b3lyp/6-31g(d,p) pop=(nbo,full) geom=connectivity
- The log file for population analysis of Borazine is available at [[13]].
- Summary Table
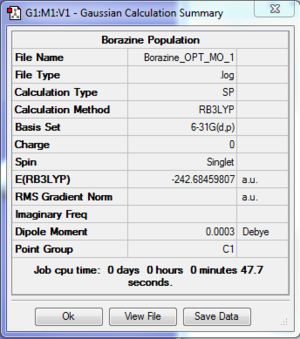
| File type | .log |
|---|---|
| Calculation Type | SP |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Final Energy | -242.68459807 a.u. |
| Gradient | a.u. |
| Dipole Moment | 0.0003 Debye |
| Point Group | C1 |
| Calculation Time | 47.7 s |
Analysis
Charge Distribution
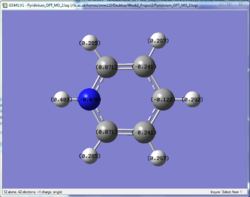
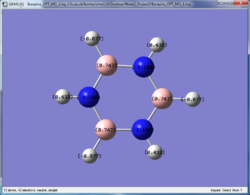
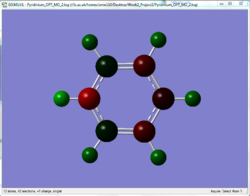
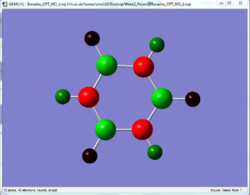
The charge distribution of the optimised molecules illustrated by colour and by number are tabulated and compared in this section.
| Benzene | Pyridinium | Boratabenzene | Borazine | |
|---|---|---|---|---|
| Distribution
By Number |

|
|

|
|
| Distribution
By Number |

|
|

|
|
From the charge distribution, it can be seen that in benzene, the charges are very evenly distributed. All the ring atoms have the same charge and the charge number is low. However, pyridinium and boratabenzene do not have even distribution due to the addition of a hetero atom into the system, although it is still aromatic.
For pyridinium, the negative charge is concentrated on the nitrogen (-0.476), as it is more electronegative, while the adjacent carbons are less negative. On the other side, in boratabenzene, boron atom carries a positive charge (+0.202), while the neighbouring carbon atoms are more negative than the carbons in benzene. This different charge distribution would generally affect the reactivity of the molecule.
Borazine has a very distinct charge distribution. It consists of 3 N and 3 B atoms connected alternatively, while all the B have a high positive charge (+0.747) while N have a negative charge (-1.102). Although it is aromatic physically stable, it would be expected to be more reactive than benzene. The higher charge on the individual atoms would make it more prone to be attacked by nucleophiles or electrophiles.
Molecular Orbitals
- MO diagram for σ and π orbitals of Benzene
- How does MOs relate to the common concepts of aromaticity?
One of concept of aromaticity is that the system tends to be more stable when the electrons are more dispersed, or more delocalised. This is observed from the MO diagrams. Usually, the MOs with lower energies are highly symmetrical, with less nodal planes and the electrons can be easily spread out in the structure.
- Comparing Molecular Orbitals
MO 7 is a σ all bonding orbital. Benzene shows a regular and symmetrical star shape, showing that the electron is distributed evenly amongst all C atoms. In pyridinium and boratabenzene, the shape was slightly distorted. In pyridinium, the MO shifted to the side of the chain with N atom and the opposite was observed in boratabenzene. MO 7 for borazine is more interesting. It formed a triangle shape, with the boron atom exposed, showing low probability of finding electrons near B. All the observation agree with the result of the charge distribution analysis in the previous section, which showed that generally electrons prefer to reside on N and avoid B.
MO 17 is a π all bonding orbital. The MO have different signs on different side of the molecule, ie above and below the plane. The trend is very similar to MO 7. MO of benzene have a regular hexagonal shape. Pyridinium MO shifted slightly towards N while boratabenzene MO shifted slightly away from B. Borazine MO also have a hexagonal shape but with 3 sides sticking out, showing the higher electron density on the N atom.
MO 22 is the LUMO of the molecule. it has 2 nodal planes, one connecting 2 opposite atoms across the ring. The other nodal plane cut through the midpoints between the 2 pairs of oppositely orientated p orbitals. Hence the MO was divided into 4 parts. In benzene, the 4 parts have similar sizes. In pyridinium, the smallest lope reside directly on N atom and the neighbouring lopes are largest. In boratabenzene, B atom is found on one of the nodal plane (having very low electron density on the atom itself) and the 2 lopes closer to B are larger in size. For borazine, 2 larger lopes are found on B while 2 smaller lopes are found on N.
Generally, in bonding MOs, the electron density tends to be higher on the N and lower than B, as compared to the C in benzene. However, most MO still have very similar shapes.
- Effect of Substitution
When one more C atoms are substituted in benzene ring, the atoms in LCAOs are no longer equivalent. Hence, the energy level of the LCAOs will start to shift up and down depends on the situation. For instance, when C is substituted by N, since N is more electronegative, the bonding orbital will shift down.
This change in energy levels in LCAOs would result in a change the energy levels of the MOs. This is because the energy of the MOs depends on the energy of LCAOs which form it and the energy gap between the new MOs also depends on the energy gap between the 2 LCAOs. For instance, if replacing C with N will lower the energy of one of the LCAO and bring 2 LCAOs closer, then the resultant bonding MO will be more stablised and have a lower energy.
The degeneracy of the molecule can be affected by the symmetry of the molecule. A more symmetric molecule tends to have a large numbers of degenerate energy levels. When C is replaced by hetero atoms, the electron distribution of one side of the ring would be different from other side, due to the difference in electronegativity. As a result, many originally degenerate levels will split to form 2 different energy levels.
In the table of MO above, the order of 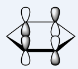
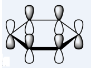
When comparing the relative energies of the MOs, it can also be found that putting N and a positive charge in the ring would largely lower the energy of the entire system, making it accept an electron more easily, (LUMO energy of pyridinium= -0.25840) while putting B with an additional electron in the ring would make adding electron to the system highly unfavourable (LUMO energy of boratabenzene= 0.23249). Although, all the rings are iso-electric and aromatic, their properties differ largely, due to the different properties of the hetero atoms added in.