Rep:Mod:Vs911
Module 1C
Conformational analysis using Molecular Mechanics
Dimerisation of cyclopentadiene
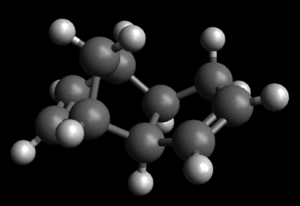 |
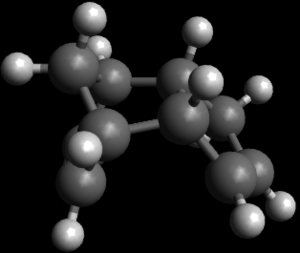 |
|
| Exo dimer of cyclopentadiene | Endo dimer of cyclopentadiene |
| Type | Exo dimer energy/kcal/mol | Endo dimer energy/kcal/mol |
| TOTAL BOND STRETCHING ENERGY | 3.54296 | 3.46704 |
| TOTAL ANGLE BENDING ENERGY | 30.77268 | 33.19239 |
| TOTAL STRETCH BENDING ENERGY | -2.08212 | -2.04136 |
| TOTAL TORSIONAL ENERGY | -2.73091 | -2.94985 |
| TOTAL OUT-OF-PLANE BENDING ENERGY | 0.01485 | 0.02211 |
| TOTAL VAN DER WAALS ENERGY | 12.80156 | 12.35647 |
| TOTAL ELECTROSTATIC ENERGY | 13.01367 | 14.18469 |
| TOTAL ENERGY | 55.37344 | 58.19074 |
Dimerization of cyclopentadiene is a bispericyclic reaction.It has both a simulataneous 4πs + 2πs and 4πs + 2πs cycloadditon. As calculated using molecular mechanics techniques,the overall energy for exo dimer of cyclopentadiene is 55.37344kcal/mol whereas the overall energy of endo dimer of cyclopentadiene is 58.19074kcal/mol.From here, it can be deduced that the exo dimer is a thermodynamic product and while endo is the kinetic product.However,endo dimer is produced exclusively due to secondary orbital overlap which gives the transition state stability which is absent in exo dimer.This favourable orbital overlap overrides the high steric repulsion which exist in endo dimer between the bridging methyl hydrogen and ring hydrogen mostly.The higher angle strain noted in endo is an evident for the high steric repulsion experienced by endo dimer. [1]
Hydrogenation of cyclopentadiene dimer
 |
 |
|
| Dihydroderivative of cyclopentadiene,(3) | Dihydroderivative of cyclopentadiene,(4) |
| Type | Dihydroderivative (3)energy /kcal/mol | Dihydroderivative (4)energy /kcal/mol |
| TOTAL BOND STRETCHING ENERGY | 3.31174 | 2.82313 |
| TOTAL ANGLE BENDING ENERGY | 31.93780 | 24.68533 |
| TOTAL STRETCH BENDING ENERGY | '-2.10243 | -1.65721 |
| TOTAL TORSIONAL ENERGY | -1.47042 | -0.37846 |
| TOTAL OUT-OF-PLANE BENDING ENERGY | 0.01325 | 0.00028 |
| TOTAL VAN DER WAALS ENERGY | 13.63625 | 10.63740 |
| TOTAL ELECTROSTATIC ENERGY | 5.11951 | 5.14702 |
| TOTAL ENERGY | 50.44571 | 41.25749 |
Hydrogenation of cyclopentadiene can occur at 2 position of double bonds which are the norbonene ring(dihydroderivative,(4)) and the 5Cring(dihydroderivative,(3)).However, from the data above, the total energy of dihydroderivative(4) 41.25749 which is much lower than total energy of dihydroderivative,(3) which is 50.44571.This hence deduce that dihydroderivative,(4) is thermodynamically stable and more favourable to form.From the data calculated,it can be seen that the the angle bending energy is higher for dihydroderivative,(3)which means the molecule has higher angle strain than (4).This can also be seen in the value of torsional and bond stretch energy.This is because dihydroderivative,(4)which lacks double bond in the norbonene ring [2] and hence, will reduce the angle strain while the the ring strain still present in (3) and higher Van der waals energy in (3) which suggest that it experience less favourable VanderWaal interaction and higher steric repulsion.Higher electrostatic energy in (4)in suggest it experiences higher electrostatic repulsion.[3]
| Structure9taxol intermediate | Structure10taxol intermediate | ||||||
|---|---|---|---|---|---|---|---|
|
|
After optimsing the 2 structures above several times(including manual edits) to identify conformers with the lowest energies, it is observed that structure 9 taxol intermediate have the C ring in a twist boat form while structure 10 taxol intermediate have its C ring in a chair like form which is in good agreement with literature[4].As for the cyclohexane ring in the taxol intermediates,each 2 chair and 2 twist boat conformation were observed during manual edits.However, opposite chair conformation was observed to produce the lowest energy for both intermediates compared to the twist boat conformation or even the other chair conformation.This is probably because due to lack of hydrogen repulsion which exist in the twist boat conformation.The overall energy of calculated using MMFF94s force field of taxol 9 taxol intermediate is 77.2939kcal/mol and structure 10 taxol intermediate is 70.82935kcal/mol.From here,structure 10 taxol intermediate is lower than structure 9 taxol intermediate which indicates it is much more thermodynamically stable and favoured to form.
The 2 alkene intermediates are known to be hyperstable alkenes which referes to the bridgehead olefin.This means alkene molecule with the bridgehead like intermediates above are stable due to bridgehead position stablisises the alkene and hence, the reactivity is slow and relatively inert.These alkene are known to have very low strain energy compared to unsaturated hydrocarbon.Bridgehead groupings in a certain medium size ring bicyclic compounds prefer to flatten, thus favoring sp2 centers. In other cases, sp3 bridgehead groups such as this prefer to pyramidalize inward[5] .The steric hindrance as in the the methyl group could also contribute to it being inert.[6]
Spectroscopic simulation using Quantum Mechanics
| Structure17taxol intermediate | |||
|---|---|---|---|
|
Structure 17 is chosen and sketched in Avagadro.It is optimised several times(including manual edits) to achieev a conformer of lowest possible energy.The total energy after optimisation calculated is 104.97883kcal/mol.In this structure, the chair conformation in the cyclohexane ring gives the lowest possible energy.The structure was then subjected to 13C and 1HNMR spectra simulation and compared with literature.[7]The solvent for all the NMR simulation in this computational exercise is using chloroform.
| 13CNMR of structure 17 | 1HNMR of structure 17 |
|---|---|
 |
 |
| Structure17calculated | Structure17reported | Difference |
| 216.11 | 218.79 | 2.68 |
| 145.13 | 144.63 | 0.5 |
| 124.68 | 125.33 | 0.65 |
| 90.65 | 72.88 | 17.77 |
| 60.64 | 56.19 | 4.45 |
| 57.05 | 52.52 | 4.53 |
| 52.477 | 48.5 | 3.977 |
| 51.55 | 46.8 | 4.75 |
| 46.7 | 45.76 | 0.94 |
| 45.95 | 39.8 | 6.15 |
| 42.17 | 38.81 | 3.36 |
| 40.57 | 35.85 | 4.72 |
| 35.33 | 32.66 | 2.67 |
| 31 | 28.79 | 2.21 |
| 29.35 | 28.29 | 1.06 |
| 29.31 | 26.88 | 2.43 |
| 27.09 | 25.66 | 1.43 |
| 26.4 | 23.86 | 2.54 |
| 22.9 | 20.96 | 1.94 |
| 19.7 | 18.71 | 0.99 |
| Structure17calculated | Structure17reported | Integration |
| 5.15 | 4.84 | dd,1H |
| 3.31-3.16 | 3.40-3.10 | m,4H |
| 3 | 2.99 | dd,1H |
| 2.73-1 | 2.8-1.35 | m,14H |
| 1.57 | 1.38 | s,3H |
| 1.49 | 1.25 | S,3H |
| 1.16 | 1.1 | s,3H |
| 0.92-0.82 | 1-0.80 | m,1H |
From the data above, it can be seen that the difference in chemical shift in the 13CNMR and 1HNMR of structure 17 between the calculated values and the reported values in the literature are fairly same although minor differences are observed.This minor difference in the chemical shift suggests that maybe another conformer of structure 17 with a lower energy might be obtained.However, with the data above, it is possible to determine the configuration of the structure 17.
Free energy for this structure,17 and also structure 18(isomeric structure of 17 with carbonyl pointing downwards) are calculated.Structure 18 was thus also modelled and computed and the free energies are obtained from the log.file.
Free energy of structure 17 =-1651.461194
Free energy of structure 18 =-1651.463260 Free energy will be appreciated if equilibrium is included in the discussion.Since,equilibrium consatant can be calculated using the equation, ∆G=-RTlnK and K is sure to be high indicating tend to form more of 18 than 17.
Analysis of the properties of the synthesised alkene epoxides
Crystal structure
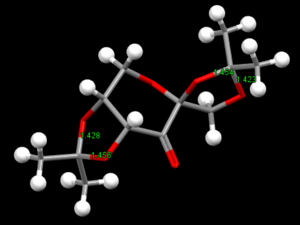 |
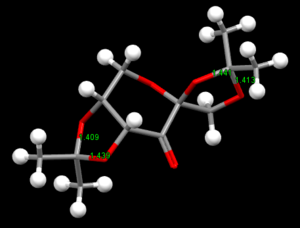 |
|
| Precursor catalyst 21-NELQEA01 | Precursor catalyst 21-NELQEA |
Conquest program was used to search the cambridge database for the structure of precatalyst 21.The structure was then opened in Mercury to analyse the CO bondlengths at the anomeric ceter.2 reference is obtained as noted as above.The bond lengths were measured for this molecule at 2 anomeric center which is found in this molecule.It can clearly be seen that some CO bond lengths arelonger/shorther than the other CO bond lengths.The values are such as 1.428 compared to 1.456 when attached to the same carbon.This probably due to the anomeric effect.It is possible the oxygen hyperconjugates with other oxygen in the molecules which causes strain in the orientation of the other CO bond which perhaps causes a longer bond.This also concluded that since one bond is longer than the other,the bonds are not identical and hence one has more double bond character than the other.
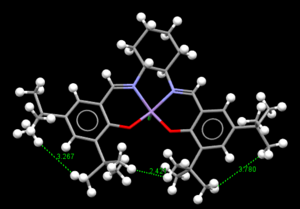 |
 |
|
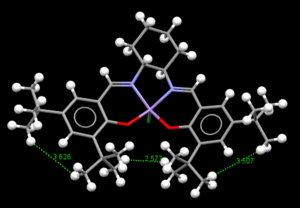
| Precursor catalyst 23-TOUNIB01 | Precursor catalyst 23-TOUNIB02 |
As for precatalyst 23,4 reference were found upon using conquest in which one of them was to wrong and the other couldn't be open in Mercury.So,the remaining 2 reference were opened in Mercury and the H-Hdistances beteween 2 adjacent tertbutyls were measured.It can be clearly seen in both cases, the H-H distance of the tert btyl nearer to center with around 2A while the H-H distance of the tert butyl from the same ring with around 3A.This probably because higher steric repulsion is present between the 2 tertbutyl group in the same ring which causes a further distances and hence, causes a stronger interaction of the tertbutyl groups in the center which cause closer approach.
The calculated NMR properties of styrene oxide and trans-Stilbenoxide
2 epoxides are chosen which are the styrene oxide and the transtillbene.Both structures were subjected 13CNMR and 1HNMR spectra simulation and are shown as below.All spectra were compared to the literature value[8] and tabulated as below.
| Structure | 13CNMR | 1HNMR |
|---|---|---|
 |
 |
 |
 |
 |
 |
| Styrene oxide reported | Styrene oxide calculated | Integration |
| 51.0 | 53.4 | 1CH3 |
| 51.1 | 54.0 | 1CH |
| 125.3 | 122.0 | 2xCH |
| 128.0 | 123.4 | 1CH |
| 128.2 | 124.13 | 2xCH |
| 137.5 | 135.13 | 1C |
| Styrene oxide reported | Styrene oxide calculated | Integration |
| 2.75 | 2.53 | dd,1H |
| 3.08 | 3.11 | dd,1H |
| 3.80 | 3.66 | dd,1H |
| 7.24-7.3 | 7.29-7.51 | m,5H |
| trans-Stillbene oxide reported | trans-Stillbene oxide calculated | Integration |
| 62.8 | 66.4 | CH |
| 125.5 | 123.2 | 4xCH |
| 128.3 | 123.5 | 2xCH |
| 128.5 | 129.2 | 4xCH |
| 137.1 | 134.1 | 2xC |
| Trans-Stilbene oxide reported | Trans-Stilbene oxide calculated | Integration |
| 3.87 | 3.54 | s,1H |
| 7.25-7.59 | 7.45-7.57 | m,10H |
As seen above,the calculated result are fairly similar to literature result.However,there are minor difference which assume that the structure need further optimisation. Although it has minor differences,the structure of both styrene oxide and trans-stilbene oxide agrees with the the calculated value and integration done.
Assigning the absolute configuration
i)The reported literature for optical rotation
| Structure | Configuration | Calculated optical rotation | Reported optical rotation |
|---|---|---|---|
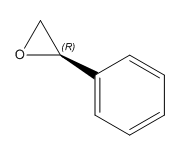 |
(R)-Styrene oxide | 30.43° DOI:10042/26493 | 32.1°[9] |
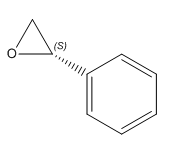 |
(S)-Styrene oxide | -30.08° DOI:10042/26495 | -33.3°[10] |
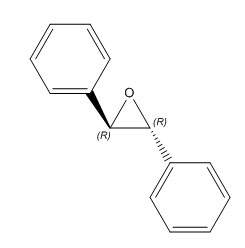 |
(R,R)-Trans-Stillbene oxide | 298.2° DOI:10042/26487 | 296.0°[11] |
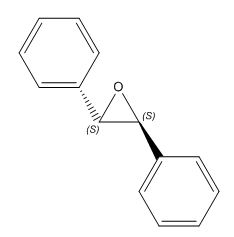 |
(S,S)-Trans-Stillbene oxide | -297.94 ° DOI:10042/26494 | -291.0 ° [12] |
Reaxys was used to find the literature value for optical rotation for the 2 epoxides chosen.The wavelength for both calculated and reported are 589nm.The calculated value is also included in this section for comparison purpose.As observed, the calculated value and literature value are fairly similar with minor difference with appropriate sign for each configuration.Eventhough Reaxys prove to be reliable in obtaining appropriate literature value but it is also observed that some of the signs are wrongly configured in some literature.
ii)The calculated chiral properties of the product
As seen above,after determining the configuration using optical rotation method,each configuration of the 2 epoxides chosen are subjected to ECD and VCD spectrum.ORD values were not included in this section as it was already discussed in the previous section.Attempts have been made to find literature spectra for this molecule using Reaxys but to no avail.If there were experimental spectra of ECD and VCD,comparison can be made and absolute configuration can be confirmed.However from the observation of the spectra obtained between (R)and(S) and (R,R)and(S,S), there is a difference in symmetry for the VCD spectrum which is confirms the different direction of polarised light during vibrational excitation and causes diffrent absolute configuration.
ii)The calculated chiral properties of the product
| Free energy of (R) styrene/Hatree | Free energy of (S) styrene/Hatree | Free energy difference/Hatree | Free energy difference/kJ/mol | K | Enantiomeric excess,ee |
| -1303.677681 | -1303.679145 | 0.001464 | 3.843732 | 0.212114236 | 17.49952522 |
| -1303.676222 | -1303.670886 | -0.005336 | -14.009668 | 284.7896586 | 99.65009231 |
| -1303.683121 | -1303.67519 | -0.007931 | -20.8228405 | 4448.430525 | 99.97752521 |
| -1303.684857 | -1303.683695 | -0.001162 | -3.050831 | 3.423830113 | 77.39515365 |
| Free energy of (R,R)trans-Stillbene /Hatree | Free energy of (S,S)trans-Stillbene/Hatree | Free energy difference/Hatree | Free energy difference/kJ/mol | K | Enantiomeric excess,ee |
| -1534.626855 | -1534.624864 | -0.001991 | -5.2273705 | 8.238471578 | 89.17569869 |
| -1534.62755 | -1534.625326 | -0.002224 | -5.839112 | 10.544478 | 91.33785001 |
| -1534.641092 | -1534.634829 | -0.006263 | -16.4435065 | 760.2177373 | 99.86863154 |
| -1534.641048 | -1534.63164 | -0.009408 | -24.700704 | 21262.62633 | 99.99529713 |
| Free energy of (S,R) cis-β-methyl styrene/Hatree | Free energy of (R,S) cis-β-methyl styrene/Hatree | Free energy difference/Hatree | Free energy difference/kJ/mol | K | Enantiomeric excess,ee |
| -3383.1851 | -3383.176944 | -0.008156 | -21.413578 | 5645.537589 | 99.98229003 |
| -3383.179671 | -3383.177857 | -0.001814 | -4.762656999 | 6.830112147 | 87.22879084 |
Since the 2 chosen epoxides are styrene oxide and trans-stillbene oxide, calculation for free energy and enantiomeric excess is carried oout on transition states for Shi epoxidation of styrene,transition states for Shi epoxidation of Stilbene and additional calculation was carried out on transition states for Jacobsen epoxidation of cis-β-methyl styrene.Ananlysis for the calculation are as below:
Analysis:
For transition states for Shi epoxidation of styrene, the overall free energy for (R)sytrene is lower than (S)sytrene which suggest that(R)sytrene oxide is favoured which is also indicated by the large K values.However, there is an exception for the first row of energy which might suggest the structure was not optimised properly.Enantiomeric excess for the second and third row agree well with literature value with literature value being 99.1ee[13]
As for Transition states for Shi epoxidation of Stilbene,the overall free energy for (R,R)transtillbene is lower than(S,S)transtillbene which suggest that epoxidation of (R,R)transtillbene is favoured.Enantiomeric excess for the third and fourth row agree well with literatur with literature value being 97.0ee[14]
As for transition states for Jacobsen epoxidation of cis-β-methyl styrene,it can be observed from the energy difference itself that (S,R)cis-β-methyl styrene is more stable and hence, more favoured to form than the other enantiomer.
Calculation for obtaining enantiomeric excess: It is asssumed X= mol fraction of the excess isomer.Hence,K=X/1-X.When this equation is rearranged, X=K/K+1 is obtained.Hence,the enantiomeric excess is obtained when (K/K+1)multiply 100 to get interms of percentage.
Investigating the non covalent interaction in the active site of the reaction

The transition state that is chosen is the transition states for Shi epoxidation of (S)styrene. As seen above, at the bond forming transition state it was fairly red and blue with a mixture of other colours which suggest it had very strong repulsion and attractive interaction which is expected because there is where bonds will be forming and breaking. As both molecule approach each other to form the transition state especially at the bond forming transition state which is also the active site,it creates a very complex system as partial bonds will be forming and breaking with more forming than breaking in between which is evident to a ring type image in the bond forming transition state.However, overall the molecule had alot of green colour which suggest it is mildly attractive which reinstate the interaction between the catalyst and styrene.
Investigating the Electronic topology(QTAIM) in the active site of the reaction
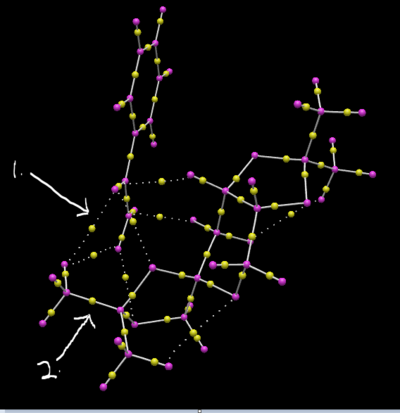
As seen above,the QTAIM calculation is carried out on the same the transition states for Shi epoxidation of (S)styrene. As seen above,no.1 corresponds to the bond critical position while the no.2 corresponds to the weak noncovalent bond.All the dotted lines corresponds to a noncovalent bond whil eall the bond line corresponds to the bond critical point.It is also observed that in a bond critical point the yellow shere is always further from an electronegative atom such as the oxygen as expected which suggest that the yellow sphere represents the electron density which moves along the bond depending on which atoms are present in the bond.
Suggesting new candidates for investigations
Reaxys was used to find an epoxide with optical rotary power more than 500 °.The epoxide chosen is trans-(2R,3R)-2-(4-methylphenyl)-3-phenyl oxirane with optical rotation of -580 °[15]and the alkene which can lead to this epoxidation chosen is 4-methyl-stilbene(CAS number: 4714-21-0)which is below.It is probably a good alkene to investigate for rotary power more thann 50 due to its simple structure.Hence,it will be easier to analyse and do comparison.

References
- ↑ Marye Anne Fox,* Raul Cardona, and N. J. Kiwiet,J. Org. Chem. 1987,52, 1469-1474
- ↑ D.Skala,J,Hanika,Petroleum and Coal,Vol 45,3-4,105-108
- ↑ http://www.ch.ic.ac.uk/local/organic/pericyclic/
- ↑ Steven W. Elmore1, Leo A. Paquette,Volume 32, Issue 3, 14 January 1991, Pages 319–322
- ↑ Alan B. McEwent and Paul von Rag& Schleyer*,J. Am. Chem. SOC. 1986, 108, 3951-3960.
- ↑ W. F. Maier, P. Von Rague Schleyer, J. Am. Chem. Soc., 1981, 103, 1891.
- ↑ Spectroscopic data: L. Paquette, N. A. Pegg, D. Toops, G. D. Maynard, R. D. Rogers, J. Am. Chem. Soc.,, 1990, 112, 277-283
- ↑ Wiles, Charlotte; Hammond, Marcus J.; Watts, Paul Beilstein Journal of Organic Chemistry, 2009 , vol. 5, art. no. 27
- ↑ Lin, Hui; Qiao, Jing; Liu, Yan; Wu, Zhong-Liu Journal of Molecular Catalysis B: Enzymatic, 2010 , vol. 67, # 3-4 p. 236 - 241
- ↑ Jensen; Kiskis Journal of the American Chemical Society, 1975 , vol. 97, p. 5825,5826
- ↑ Solladie-Cavallo, Arlette; Diep-Vohuule, Anh; Sunjic, Vitomir; Vinkovic, Vladimir Tetrahedron: Asymmetry, 1996 , vol. 7, # 6 p. 1783 - 1788
- ↑ Read; Campbell Journal of the Chemical Society, 1930, p. 2377Nature (London, United Kingdom), 1930 , vol. 125, p.16
- ↑ Lin, Hui; Qiao, Jing; Liu, Yan; Wu, Zhong-Liu Journal of Molecular Catalysis B: Enzymatic, 2010 , vol. 67, # 3-4 p. 236 - 241
- ↑ Wong, O. Andrea; Wang, Bin; Zhao, Mei-Xin; Shi, Yian Journal of Organic Chemistry, 2009 , vol. 74, # 16 p. 6335 - 6338
- ↑ Dansette P.M. et al. Tetrahedron,1976,vol36,pg2071-2074








