Rep:Mod:Vladimir
Molecular Optimisation
Different molecular analysis
Molecule: NH3
Bond angle = 109.471
Bond lenght = 1.3 angstrom
Calculation method: RB3LYP
Basis Set: 6-31G(d,p)
Final Energy (au):-56.55776871
RMS Gradient: 0.05399560
Point Group: C3V
Charge on N atom = -1.125 Charge on H atoms = +0.375
I would expect a negative charge on N because it is a more electronegative element than H.
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES
Ammonia |
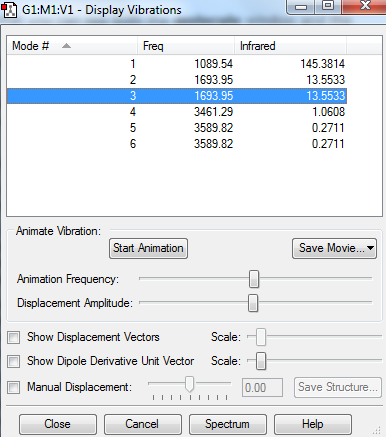
From the 3N-6 rule I would expect 3*4-6=12 vibrations in these molecule. As seen from the list of vibrations above this rule is followed. Modes 2-3 and 5-6 are degenerate. They vibrate with the same frequency and thus the same energy. Modes 1,4,5,6 are stretching. Modes 2 and 3 are bending. Mode 4 is highly symmetric. Mode 1 is called the umbrella vibration mode. In the experimental spectrum of gaseous ammonia you would expect to see only 2 modes, because modes 4,5 and 6 have a very low intensity and as a result of noise on the spectrum these would not be seen. Also, modes 2 and 3 are degenerate so only one signal would be seen for these two as the spectrometer cannot resolve modes of the same frequency.
Molecule: H2
Bond angle = N/A
Bond lenght = 0.74279 angstrom
Calculation method: RB3LYP
Basis Set: 6-31G(d,p)
Final Energy (au):-1.17853936
RMS Gradient: 0.00000017
Point Group: Dinfh
Charge on H atoms = no charge, neutral molecule
Item Value Threshold Converged?
Maximum Force 0.000000 0.000450 YES
RMS Force 0.000000 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000001 0.001200 YES
Hydrogen |
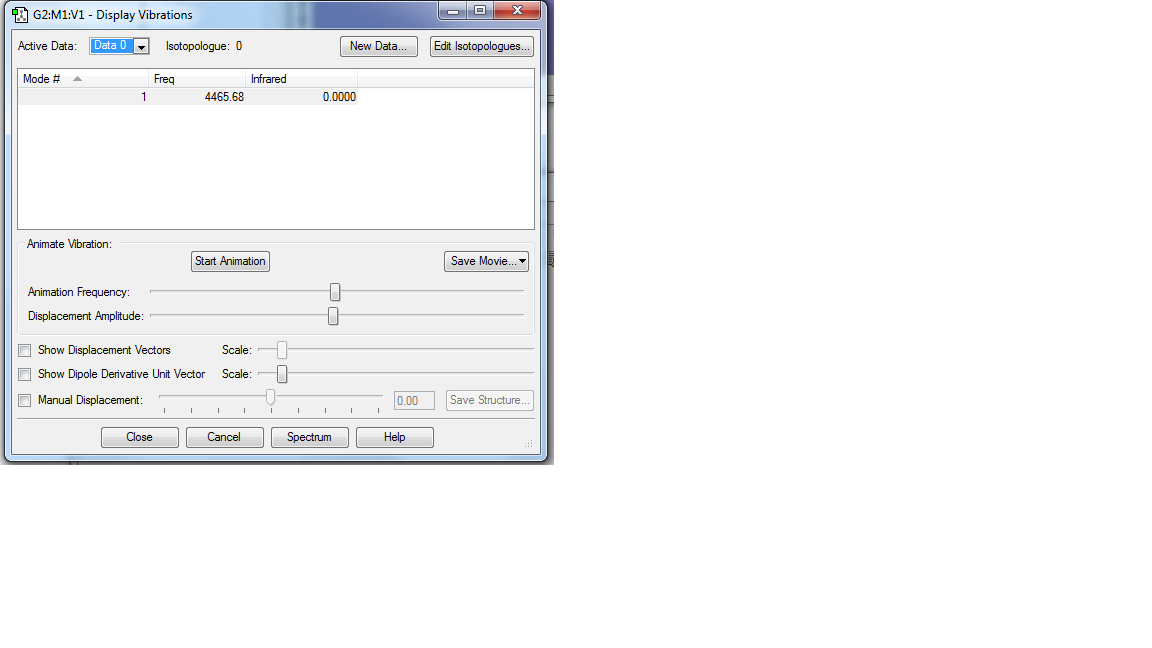
Only one vibration is observed which is IR inactive because there is no net change in dipole.
Molecule: N2
Bond angle = N/A
Bond lenght = 1.10550 angstrom
Calculation method: RB3LYP
Basis Set: 6-31G(d,p)
Final Energy (au):-109.52412868
RMS Gradient: 0.00000060
Point Group: Dinfh
Charge on H atoms = no charge, neutral molecule
Item Value Threshold Converged?
Maximum Force 0.000001 0.000450 YES
RMS Force 0.000001 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000000 0.001200 YES
Nitrogen |
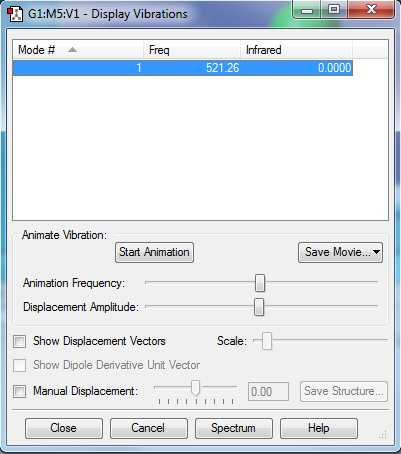
Only one vibration is observed which is IR inactive because there is no net change in dipole.
Total Energy Calculation
N2 + 3H2 -> 2NH3
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]=
2*E(NH3)= -113.11553742 E(N2)= -109.52412868 3*E(H2)= -3.53561808
ΔE=-113.11553742-[-109.52412868+-3.53561808]= -0.05579066 au
ΔE=-148.12 kJmol-1
According to an online resource the reaction is indeed exothermic, but releases only 92.4 kJ mol-1 of energy[1]
This is good as the value confirms that the reaction is exothermic; however, the calculations account only for the energy of the reactants and products. In actual fact the environment where the reaction was carried out matters and the way the molecules interact.
Molecule: Cl2
Bond angle = N/A
Bond lenght = 2.04101 angstrom
Calculation method: RB3LYP
Basis Set: 6-31G(d,p)
Final Energy (au):-920.34987875
RMS Gradient: 0.00011755
Point Group: Dinfh
Charge on Cl atoms: Neutral molecule thus no charge
Item Value Threshold Converged?
Maximum Force 0.000204 0.000450 YES
RMS Force 0.000204 0.000300 YES
Maximum Displacement 0.000566 0.001800 YES
RMS Displacement 0.000801 0.001200 YES
Chlorine |
Similarly to N2 only one vibration is observed which is also IR inactive because there is no net change in dipole.
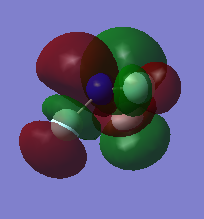
Molecular Orbitals of Cl2
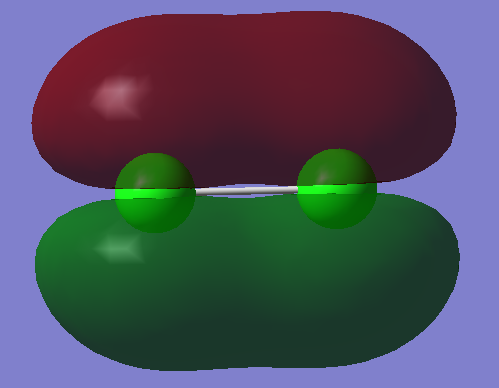
1)
In this example two 1s AOs contribute to the MO. However, their energy is so small that no overlap of orbitals is seen. Thus this orbital is non-bonding.
2)
In this example two 3py orbitals overlap in phase in order to form a pi orbital. Such configuration is stable and thus has a low energy. This orbital is bonding.
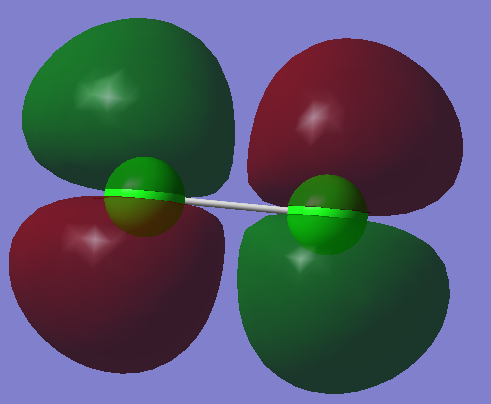
3)
In this example two 3py orbitals overlap out of phase to form a pi orbital which is of a higher energy than in the previous example where the overlap was in phase. This orbital is anti-bonding.
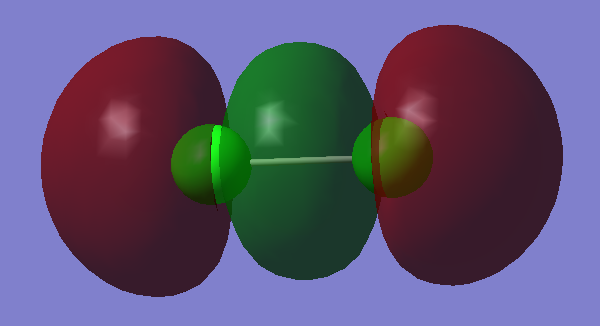
4)
In this example two 3pz orbitals overlap in phase to form a pi orbital of low energy. The geometry of the overlap is different to the overlap of 3py orbitals in phase and in fact the overlap in this example produces a configuration of a lower energy. This orbital is bonding.
5)
Finally, here 3pz orbitals overlap out of phase forming the LUMO with a high energy. It is the only molecular orbital which does not have electrons and has the highest energy at the same time. This orbital is anti-bonding.
References
- ↑ The energy of the Haber Process Reaction http://www.ausetute.com.au/haberpro.html