Rep:Mod:Vishali Pala16
Revision
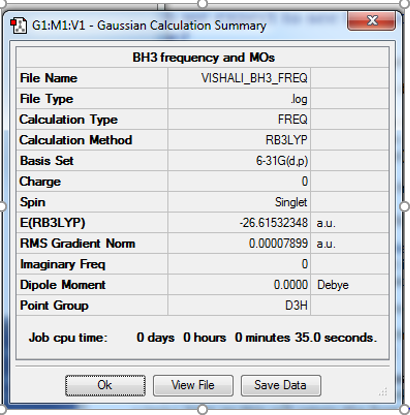
BH3
Optimisation
The optimisation method used was B3LYP. The basis set was 6-31G (d,p). Below are summary and item tables resulting from the optimisation. It can be seen that the job has fully converged and the optimisation has completed.
Item Value Threshold Converged? Maximum Force 0.000158 0.000450 YES RMS Force 0.000079 0.000300 YES Maximum Displacement 0.000622 0.001800 YES RMS Displacement 0.000311 0.001200 YES
Frequency Analysis
The link to my log file is here.
The low frequencies obtained are also included:
Low frequencies --- -0.2456 -0.1129 -0.0054 44.0270 45.1846 45.1853 Low frequencies --- 1163.6049 1213.5924 1213.5951
Jmol Image
An interactive image of borane is shown:
Optimised Borane Molecule |
Vibrational Analysis
Below is a table summarizing the molecular vibrations found in borane. It can be seen that the stretches occur at higher wavenumbers than the bends and are therefore associated with higher energies. This is typical when studying the same bond.
| Wavenumber cm-1 | Intensity (arbitrary units) | Symmetry | IR active? | Type |
|---|---|---|---|---|
| 1164 | 92 | A2 ' ' | Yes | Out of plane bend |
| 1214 | 14 | E' | Very slight | Asymmetric bend |
| 1214 | 14 | E' | Very slight | Symmetric bend |
| 2580 | 0 | A1' | No | Symmetric stretch |
| 2713 | 126 | E' | Yes | Asymmetric stretch |
| 2713 | 126 | E' | Yes | Asymmetric stretch |
Ng611 (talk) 14:07, 21 May 2018 (BST) For your entry at 1214 cm-1, how can the two degenerate orbitals have different vibrational modes? Rest of the analysis was good however, well done.
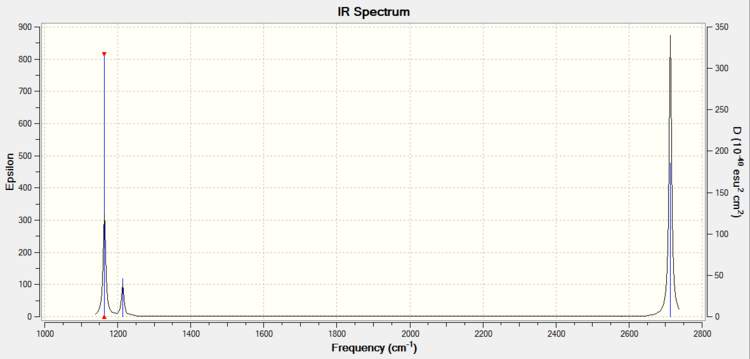
The computed IR spectrum is shown:
There are six vibrations but only three peaks in the spectrum. This is due to:
1) Degeneracy - there are two pairs of vibrations occurring at the same energy. These four vibrations give rise to only two degenerate peaks ( 1214 and 2713 cm-1)
2) Dipole Moment - the selection rule for an IR absorption is that there must be a change in the dipole moment. The vibration at 2580 cm-1 is a symmetric stretch and so has no change in dipole moment. This is confirmed by studying the D3h character table. Symmetry labels that lead to infrared activity are associated with the co-ordinate axes Tx,Ty and Tz. Only E' and A2' ' correspond to these so are expected to be IR active. A1' is therefore not IR active and not observed.
Molecular Orbital Diagram

The molecular orbital diagram for borane is shown on the right.1 The predicted orbitals from a linear combination of atomic orbitals are given alongside the computed orbitals. The core 1s orbital on boron is too deep in energy to interact with hydrogen, and therefore remains non-bonding. The valence orbitals on B are 2s and 2p. For interacting orbitals that are initially further apart in energy, there is an unequal contribution from the B and H orbitals. This is seen with the 2a1' which has a larger contribution from hydrogen, compared to the 3a1' which has a larger contribution from boron. On the other hand, 1e' and 2e' are formed from fragment orbitals that are closer in energy and so contributions from boron and hydrogen are more equal. The highest occupied molecular orbital is the 1e'. The lowest unoccupied molecular orbital is the 1a2' ' which is in fact non-bonding. This orbital is used to accept electron density from a lewis base.
We can compare the orbitals predicted from LCAO and the ones produced computationally. The occupied orbitals show good agreement, both in terms of the overall shape and phase. However, there are discrepancies in some of the unoccupied orbitals. For example, the 3a1' orbital shows a much larger contribution from the hydrogen atoms than expected. Boron is less electronegative and therefore would be expected to have a greater contribution to the higher energy antibonding orbital. This shows that computational prediction of occupied orbitals is much more accurate than for unoccupied orbitals which can be much more diffuse.
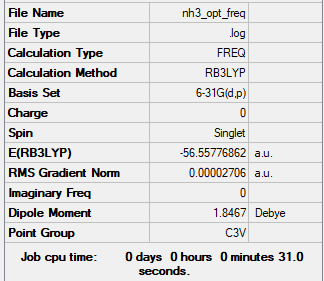
NH3
Optimisation
The optimisation method used was B3LYP. The basis set was 6-31G (d,p). Below are summary and item tables resulting from the optimisation. It can be seen that the job has fully converged and the optimisation has completed.
Item Value Threshold Converged? Maximum Force 0.000060 0.000450 YES RMS Force 0.000040 0.000300 YES Maximum Displacement 0.000369 0.001800 YES RMS Displacement 0.000162 0.001200 YES
Frequency Analysis
The link to my log file is here
The low frequencies obtained are also included:
Low frequencies --- -32.4235 -32.4224 -11.4276 -0.0044 0.0115 0.0476 Low frequencies --- 1088.7628 1694.0251 1694.0251
Jmol Image
An interactive image of ammonia is shown:
Optimised Ammonia Molecule |
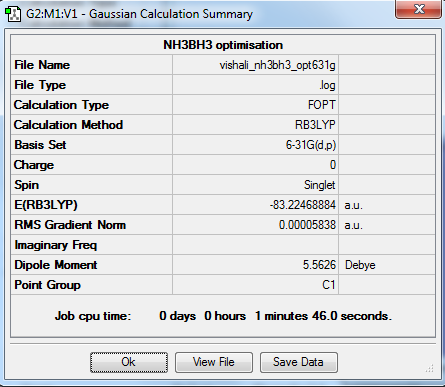
NH3BH3
Optimisation
The optimisation method used was B3LYP. The basis set was 6-31G (d,p). Below are summary and item tables resulting from the optimisation. It can be seen that the job has fully converged and the optimisation has completed.
Item Value Threshold Converged? Maximum Force 0.000138 0.000450 YES RMS Force 0.000038 0.000300 YES Maximum Displacement 0.001018 0.001800 YES RMS Displacement 0.000223 0.001200 YES
Frequency Analysis
The link to my log file is here
The low frequencies obtained are also included:
Low frequencies --- -18.7482 0.0005 0.0012 0.0013 13.0949 18.9696 Low frequencies --- 262.6515 631.2075 638.2865
Jmol Image
An interactive image of the adduct is shown:
Optimised Ammonia-Borane Adduct |
Ammonia-Borane Association Energy
We can compare the energies obtained for borane, ammonia, and the acid-base adduct to find the corresponding association energy. This is calculated below:
E(NH3) = -56.55777 a.u.
E(BH3) = -26.61532 a.u.
E(NH3BH3) = -83.22469 a.u.
ΔE = E(NH3BH3)-[E(NH3)+E(BH3)]= -0.05160 a.u. = -136 kJ/mol
Ng611 (talk) 14:11, 21 May 2018 (BST) You got the right value in hartrees (0.05160 a.u., which comes to about -135.47 kj/mol, but you rounded to 136 instead of 135 (the correct answer). So close!
We can understand this by comparing to a few typical bond dissociation energies. C-C, B-H and N-H bonds have a strength of 618, 345 and 339 kJ/mol respectively.2 Based on this, the B-N dative covalent bond is relatively weak. Nevertheless, the energy is negative and so the association is favourable.
Ng611 (talk) 14:11, 21 May 2018 (BST) Good comparison and good reference to literature.
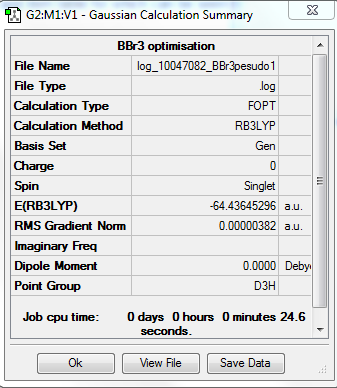
BBr3
Optimisation
The optimisation method used was RB3LYP. The basis set was Gen. Below are summary and item tables resulting from the optimisation. It can be seen that the job has fully converged and the optimisation has completed.
Item Value Threshold Converged? Maximum Force 0.000008 0.000450 YES RMS Force 0.000005 0.000300 YES Maximum Displacement 0.000036 0.001800 YES RMS Displacement 0.000023 0.001200 YES
Frequency Analysis
The link to the log file is at DOI:10042/202361 . The frequency analysis is at DOI:10042/202373 .
The low frequencies obtained are also included:
Low frequencies --- -0.0137 -0.0064 -0.0046 2.4315 2.4315 4.8421 Low frequencies --- 155.9631 155.9651 267.7052
Jmol Image
An interactive image of the molecule is shown:
Optimised Boron Tribromide Molecule |
Aromaticity Project
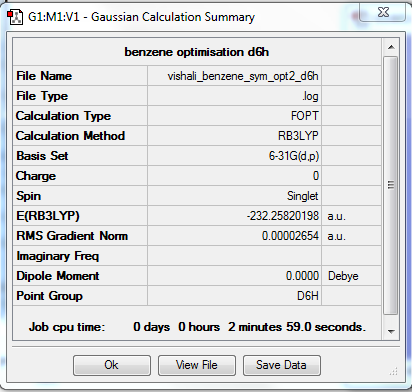
Benzene
Optimisation
The optimisation method used was B3LYP. The basis set was 6-31G (d,p). Below are summary and item tables resulting from the optimisation. It can be seen that the job has fully converged and the optimisation has completed.
Item Value Threshold Converged? Maximum Force 0.000074 0.000450 YES RMS Force 0.000019 0.000300 YES Maximum Displacement 0.000111 0.001800 YES RMS Displacement 0.000051 0.001200 YES
Frequency Analysis
The link to my log file is here.
The low frequencies obtained are also included:
Low frequencies --- -0.0087 -0.0041 -0.0040 12.5838 12.5838 16.4940 Low frequencies --- 414.3526 414.3526 621.2606
Jmol Image
An interactive image of benzene is shown below. It can be seen to have D6h symmetry.
Optimised Benzene Molecule |
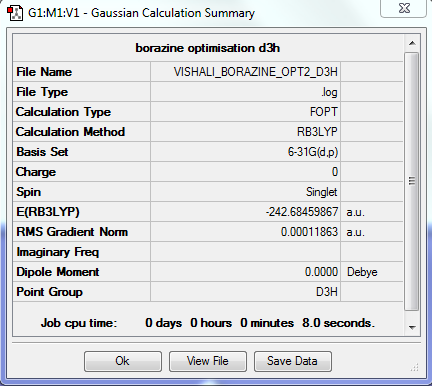
Borazine
Optimisation
The optimisation method used was B3LYP. The basis set was 6-31G (d,p). Below are summary and item tables resulting from the optimisation. It can be seen that the job has fully converged and the optimisation has completed.
Item Value Threshold Converged? Maximum Force 0.000122 0.000450 YES RMS Force 0.000053 0.000300 YES Maximum Displacement 0.000484 0.001800 YES RMS Displacement 0.000175 0.001200 YES
Frequency Analysis
The link to my log file is here.
The low frequencies obtained are also included:
Low frequencies --- -14.2497 -13.9573 -10.8574 -0.0110 0.0327 0.0562 Low frequencies --- 289.1151 289.1264 404.3008
Jmol Image
An interactive image of borazine is shown below. It can be seen to have D3h symmetry.
Optimised Borazine Molecule |
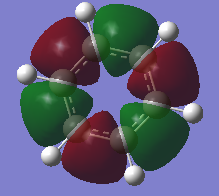
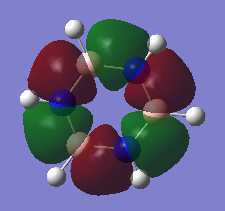
Natural Bond Orbital (NBO) Analysis of Charge Distribution on Benzene and Borazine
| Benzene | Borazine |
|---|---|
 |
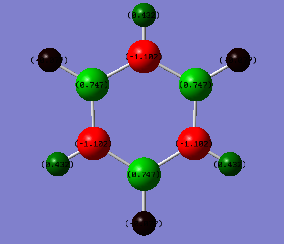
|
| Carbon = -0.24 | Boron = 0.75 |
| Hydrogen = 0.24 | Nitrogen= -1.10 |
| - | Hydrogen bonded to nitrogen = 0.43 |
| - | Hydrogen bonded to boron = -0.08 |
A range of colours have been used to qualitatively demonstrate the charge distribution on a scale of -1.10 to +1.10. Red is indicative of negative charge, and green of positive charge. Black lies between these two extremes, and therefore the brighter coloured atoms have a greater charge, whether it be positive or negative. On visual inspection, it is clear than there is a more polarized charge distribution in borazine, compared to benzene in which the atoms are darker coloured.
We can also quantitatively analyse the charge distribution. Both molecules are neutral overall and this is confirmed by addition of the charges on each atom, giving a net charge of zero. The C-H bonds in benzene are polarized towards carbon, as expected from its greater electronegativity. The distribution is overall symmetric across all C-H bonds. However, the charge distribution in borazine is more varied. Boron has the most positive charge, which correlates with it being the most electropositive. This has impacted the charge on the hydrogen atoms bound to it, which now have a negative charge. In contrast, hydrogen atoms bound to the electronegative nitrogen atoms have a positive charge.
Ng611 (talk) 14:14, 21 May 2018 (BST) Well done for explaining that the partial charges sum to 0 (very few people pointed that out and it is important.). What can you say about charges on atoms related by symmetry?
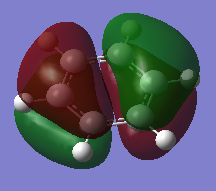
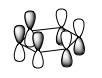
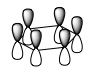
Comparison of Selected Benzene and Borazine Molecular Orbitals
Ng611 (talk) 14:18, 21 May 2018 (BST) Excellent analysis - the LCAO decomposition is a very useful tool and helps a grat deal with your analysis (remember to include axes though so it's absolutely clear that you understand what you mean when you refer to '2pz orbitals')
Discussion of Aromaticity
Aromaticity is a concept that is continuously being re-evaluated. Aromatic compounds have gained interest for their unusual stability stemming from their 'resonance energy'. This has been attributed to their tendency to undergo substitution reactions which conserve aromaticity rather than addition reactions which break it and result often in a higher energy system.3
Huckel theory has been used extensively to describe aromaticity. These systems are planar with a cyclic array of p orbitals and 4n + 2 pi electrons. Benzene and borazine both comply with these 'rules': C, N and B are all sp2 hybridized with an orthogonal p orbital and have six pi electrons. Whereas in benzene these derive from each carbon atom contributing a single electron, in borazine the boron atom is formally electron deficient. Therefore it is each nitrogen atom that contributes a lone pair of electrons to form the six pi electron system. These orbitals overlap to give areas of electron density above and below the ring. The idea of delocalization can be visualised in the molecular orbitals which are spread over several atoms.
Studies of the bond lengths of aromatic cores have revealed that they are intermediate between observed single and double bond lengths, indicating delocalization. Aromatic compounds also display unusual behaviour under externally applied magnetic fields. A ring current is induced which shields atoms internal to the ring and deshields external atoms. These atoms can be studied by NMR as their chemical shifts are observed upfield and downfield respectively. 3
Ng611 (talk) 14:22, 21 May 2018 (BST) Are there any NMR studies on Borazine in the literature that you could include here?
More recently, it has since been found that planarity is not a necessity for aromaticity. The concept of aromaticity has been associated with the overlap of pz atomic orbitals, resulting in delocalized pi systems. The sigma framework has previously been thought to be irrelevant, however it is now thought that this also has importance.3
Ng611 (talk) 14:21, 21 May 2018 (BST) Good discussion. I would go into a bit more detail in your final two paragraphs though - these are really interesting ideas and a more thorough discussion would strengthen this section further.
Ng611 (talk) 14:24, 21 May 2018 (BST) A very solid report, very well done. You dropped a few marks due to silly mistakes (rounding up when you should have rounded down, for example) - remember to carefully proofread your work! Besides these, this was a very good report - well done.
References
1. www.huntresearchgroup.org.uk/teaching/teaching_comp_lab_year2a/Tut_MO_diagram_BH3.pdf, (accessed May 2018)
2. Luo, Y. R., Comprehensive Handbook of Chemical Bond Energies, CRC Press, Boca Raton, FL, 2007.
3. M. Palusiak and T. M.Krygowski, Chem. Eur. J., 2007, 13, pp. 7996-8006.