Rep:Mod:Victoria4
Diels Alder Investigations
The Deils Alder Reaction is a pericyclic reaction. electrons fomr conjugated pi systems cyle round a ring like transition state using pi bonds to form new sigma bonds. The following investgation is into this reacton.
The first task was to model this reaction:


The first objective was to model cis butadiene on Gaussview and clean and optimise the structure. This structure had to be cleaned because i found that without cleaning the optimisation produced a cis butadiene which was non-planar, however all 4 carbon atoms in the moelcule are sp2 hybridised and therfore have trignoal planar sp2 orbitals for interaction, the results butadiene moelcule should therefore be planar. The structure was optimised using a semi empircal method, AM1. the enry for the cis butadieen was 0.04879719 hatress.. (the value when it was optimised with the HF/3-21G method was -154.05394316 hatrees). The structure had a point group of C2v.
The HOMO and LUMO Molecular Orbitals were then computed.
 |
 |
 |
 |
The LUMO MO was found to be symmetric as a plane of symmetry exists (the xz plane). The HOMO is not symmetric, its anti symertic becasue although the shapes of the orbital are symetric in the xz plane the phase of the orbitals is not symmetric.The energy of the HOMO was -3.4381 Hatrees and the LUMO 0.01707 Hatrees (when calculated by the HF/3-21G method the HOMO had an energy of -0.32599 and the LUMO 0.12366 Hatrees). The MOs show the pi sysmtes in both the ethene and the cis butadiene. It6 is the voerlap and interaction of these pi orbitals that will create the sigma bonds in the reaction.
The next task was to compute the transition state for this reaction. The areas of bond forming and at the ends of the pi conjugated system of the butadiene and each carbon fo the ethene and also the single bond between the two pi bonds of the cis butadiene which will become a pi bond. The areas of bond breaking are each pi bond of the cis butadien which willl both become sigma bonds in the product and the pi bond of the ethene structure which will become a sigma bond. In order to compute the transition state accurately it is important to have a good idea of how the transisiton state will look. In the first calcualtions I carried out to find the transition state (using the TS(berny) algorithim) the sigma bond forming areas were modelled to be 2.0A apart. The calculation failed. This is due to the fact that bonds in Gaussian are based on a pre-determined length between atoms. the molecules for my reaction were too close together which means that gaussian treated them as if they were already bonded and therefore no transistion state was found.

The calculation was rerun with longer bond lengths (2.2A). This calcualtion did find a transiton state with an imaginary frequency which shows the bonds forming. This is proof that the optimization foud a transition state as the single imaginary frequnecy represents symmetric bonds being formed. The energy of this transtion state was 0.20534216 hatrees. The imagiary frequency was found at -319.98cm-1 with an intensity of 0.3131.
The bond lengths for the fomring sigma bonds were 2.14354A. The length between the two Sp2 carbons (pi bond) was 1.33781A and the Sp3-Sp3 bond length for a sigma C-C bond was 1.56954. This shows us that in the transition state the bonds are not fully fomred. Neverthe;ess, the bond is a shorter distance than the sum of two Carbon Van Der Waals radii (1.7A) so clearly the interactiion here involved the overlap of the 'effective radii' of the carbon atoms involved. Also, they have a very long bond length compared to the bond length of the other carbon carbon bonds in the molecule. The bond length of a C-C sigma bond (sp3 bond)is 1.54A and the bond length of a C=C double bond (sp2 bond) is 1.34A. In the transition state the sp2sp2 bond and the sp3sp3 bond are far closer to their literature values than the sp3-sp3 sigma bonds that are forming. This suggests that the reaction proceeds by lengthening the ethene bond to be closer to the length of a sigma bond and shortening the simga bond in the cis butadiene before fomring the new sigma bonds and breaking the pi bonds in the diene (which in the transisition state are 1.48325A).
The Transition states imaginary frequency shows that the formation of the sigma bonds is synchronous, they occur at the smae time, the molecule vibrateds in a symmetric fashion. teh smallest positive frequency is at 141.04cm-1 (with a small intensity of 0.4198). This frequency shows the sigma bonds forming in a non synchronous fashion.


 |
 |
 |
 |
The computed MOs for the Diels Alder reaction of cis-butadiene and ethene show the HOMO as anti-symmetric (a) and the LUMO as symmetric (s). The HOMO lcearly shows the bonding interaction betweeen the two molecules. It is within this 'bonding' region that we see the new sigma bonds being formed. When a reaction occurs the MOs which are 'mixing' must have a similar symmerty, ie, they must both be symmetric or both be anti symmetric. The LUMO of the cis butadiene and the HOMO of the ethene are therefore 'allowed' to mix as they both have symmetric symmetry, and the HOMO of butadiene and the LUMO of ethene are allowed to mix because they are both anti-symmetric. the matching symmetries of the HOMO-LUMO means the reaction is 'allowed' to proceed where these orbitals are mixing.
The Diels Alder reaction of cyclohexa-1,3-diene reaction with maleic anhydride
This reaction is another Diels alder reaction. The task in this section is to examine the two routes by which the reaction could proceed, i.e. via the EXO or ENDO reaction.
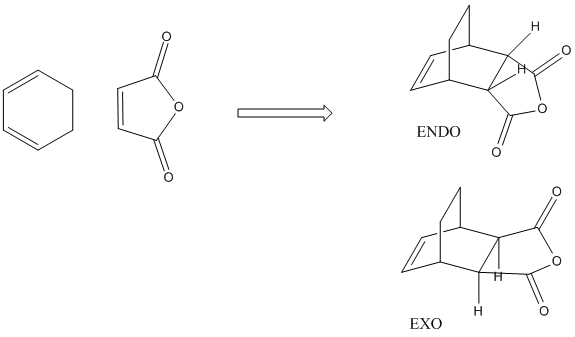
An approximate guess at the transition state for each conformer was modelled on Gaussview. I order to optimise the approximate transisito states I chose to use the forzen coordinate method. The conformers were modelled with the two molecules around 2.2A aprat and this is the length at which the bodns were set.
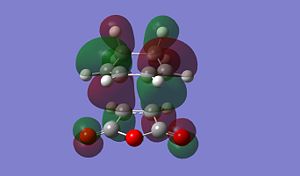
The result of this reaction is that primarily the endo product is formed, despite the product being less stable than the exo product. In the exo product we can see that there is less steric strain between the Hydrogen group on the sp3 carbon on diene and the H on the maleic anhydride. In the exo form the gap between these two groups is much larger than in the endo form in which the hydrogen atoms are pointing towards each other, inducing steric strain. The more important origin of steric strina is the strain between, in the endo, the C=OOC=C and -CH-CH- group on the diene and in the exo, the C=OOC=C and the -CH2-CH2- gourp on the diene. as we can see form the MO diagrams in the exo form the larger CH2-CH2 gourp has tetrahederal carbon centres whose H atoms point directly towards the large electron dense Oxygen atoms on the anhydride. This cuases unfavourbale steric interactions. In the endo form however the Oxygen rich fragemtn is onyl interacting with -CH-CH- so there is far less steric interaction. This means the EXO form is more sterically strained.
From the MO diagrmas we can see that there are two 'planes' of MOs in the molecule. In both confromers we can see that there is overlap betwen the -HC=CH- fragemnt of the diene moelcule (this is the pi bond which is being formed in the reaction) and the C(=O)OC(=O) fragment of the maleic anhydride. this is 'Secondary Orbital Overlap' that stabilises the endo transition state and causes it to be the predominant product. In the endo from we can see that the two 'planes' of orbtials are more 'lined up' where as in the exo from these orbitals are more staggered. The lining up of the orbitals int he endo form causes an interaction which is favourable and stabilises the transition state. the lack of this orbital overlap in the exo form menas it is not stabilised in this way. The secondary orbitla overlap stabilisation has a greater effect than the steric destabilisation so the endo form has a more stable transisiton state.