Rep:Mod:VSJ8936
NH3 molecule
Further details of NH3
optimised N-H bond distance = 1.01798 angstrom
H-N-H bond angle = 105.741°
| Calculation method | Basis set | Final energy, E(RB3LYP), in atomic units | RMS Gradient Norm in atomic units | Point group |
|---|---|---|---|---|
| RB3LYP | 6-31G(d,p) | -56.55776873 | 0.00000485 | C3v |
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES Predicted change in Energy=-5.986276D-10
test molecule |
Vibrations of NH3
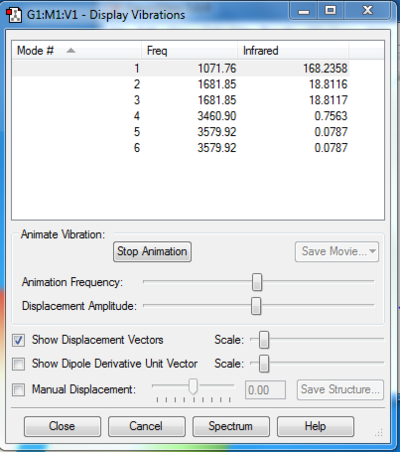
1. How many modes do you expect from the 3N-6 rule?
2. Which modes are degenerate (ie have the same energy)?
3. Which modes are "bending" vibrations and which are "bond stretch" vibrations?
4. Which mode is highly symmetric?
5. One mode is known as the "umbrella" mode, which one is this?
6. How many bands would you expect to see in an experimental spectrum of gaseous ammonia?
1. An ammonia molecule has 4 atoms per molecule, so via the 3N-6 rule, the expected number of modes is 6. 2. Modes 2 and 3 have the same energy while modes 5 and 6 also have the same energy. 3. Modes 1, 2 and 3 correspond to bending vibrations and modes 4, 5 and 6 correspond to bond stretch vibrations. 4. Mode 4 (one of the 'bond stretch' modes) is highly symmetric. 5. Mode 1 is known as the 'umbrella' mode, due to the way the molecule bends. 6. 3 bands would be expected to be seen.
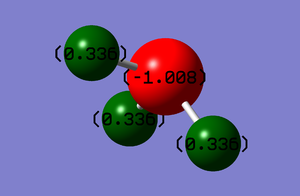
Charge distribution in NH3
The N atom has a charge of -1.008 while each H atom has a charge of 0.336. This is as expected, because nitrogen is much more electronegative than hydrogen and therefore tends to pull the electrons towards itself, thus making it more negative.
N2 molecule
Further details of N2
optimised bond distance = 1.10550 angstrom
bond angle = 180.000°
| Calculation method | Basis set | Final energy, E(RB3LYP), in atomic units | RMS Gradient Norm in atomic units | Point group |
|---|---|---|---|---|
| RB3LYP | 6-31G(d,p) | -109.52412868 | 0.0000060 | DinfH |
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES Predicted change in Energy=-3.400965D-13
test molecule |
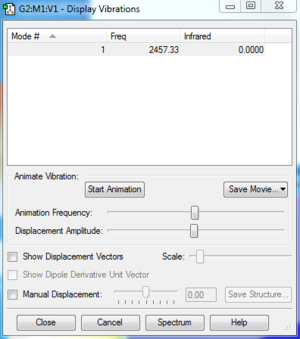
Vibrations of N2
INDEPENDENT WORK
1. How many modes do you expect from the 3N-5 rule?
2. Which modes are degenerate (ie have the same energy)?
3. Which modes are "bending" vibrations and which are "bond stretch" vibrations?
4. Which mode is highly symmetric?
5. One mode is known as the "umbrella" mode, which one is this?
6. How many bands would you expect to see in an experimental spectrum of gaseous nitrogen?
1. 1 vibrational mode is expected, because it only consists of 2 atoms. 2. N/A 3. A nitrogen molecule only has one mode; it is the 'bond stretch' mode. 4. N/A 5. N/A 6. 0 bands would be seen; this is because the frequency of its vibration has an IR value of 0, which means that its vibrational band will not show up.
Charge distribution in N2
Each N atom has a charge of 0 because both have the same electronegativity, and so each pulls the electrons towards itself equally and therefore the overall charge is 0.
H2 molecule
Further details of H2
optimised bond distance = 0.74279 angstrom
bond angle = 180.000°
| Calculation method | Basis set | Final energy, E(RB3LYP), in atomic units | RMS Gradient Norm in atomic units | Point group |
|---|---|---|---|---|
| RB3LYP | 6-31G(d,p) | -1.17853936 | 0.00000017 | DinfH |
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES Predicted change in Energy=-1.164080D-13
test molecule |
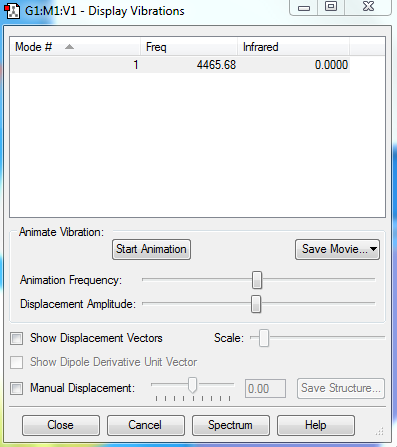
Vibrations of H2
INDEPENDENT WORK
1. How many modes do you expect from the 3N-5 rule?
2. Which modes are degenerate (ie have the same energy)?
3. Which modes are "bending" vibrations and which are "bond stretch" vibrations?
4. Which mode is highly symmetric?
5. One mode is known as the "umbrella" mode, which one is this?
6. How many bands would you expect to see in an experimental spectrum of gaseous hydrogen?
1. 1 vibrational mode is expected, because it only consists of 2 atoms. 2. N/A 3. A hydrogen molecule only has one mode; it is the 'bond stretch' mode. 4. N/A 5. N/A 6. 0 bands would be seen; this is because the frequency of its vibration has an IR value of 0, which means that its vibrational band will not show up.
Charge distribution in H2
The charge on each H atom is 0 because both atoms have the same electronegativity; they pull the electrons towards themselves equally, and therefore the overall charge on each atom is 0.
Reaction energy between H2, N2 and NH3
N2 (g) + 3H2 (g) ⇌ 2NH3 (g)
E(NH3)= -56.55776873 au
2*E(NH3)= -113.1155375 au
E(N2)= -109.52412868 au
E(H2)= -1.17853936 au
3*E(H2)= -3.53561808 au
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -0.05579074 au = -146.4785879 kJ mol-1
The negative sign in ΔE indicates that the forward reaction is exothermic, which means that more energy is given out than taken in overall.
Nitrogen gas and hydrogen gas together have a more negative energy than ammonia, therefore the gaseous reactants are more stable than the product (ammonia).
Project molecule -PH5
Further details of PH5
optimised P-H bond distance = 1.35000 angstrom.
H-P-H bond angle 1 = 90.000°
H-P-H bond angle 2 = 120.000°
| Calculation method | Basis set | Final energy, E(RB3LYP), in atomic units | RMS Gradient Norm in atomic units | Point group |
|---|---|---|---|---|
| RB3LYP | 6-31G(d,p) | -344.25491049 | 0.00000471 | D3H |
Item Value Threshold Converged? Maximum Force 0.000009 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000055 0.001800 YES RMS Displacement 0.000022 0.001200 YES Predicted change in Energy=-1.032823D-09
test molecule |
Vibrations of PH5
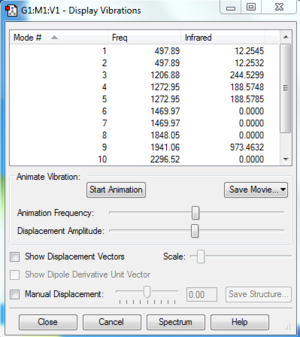
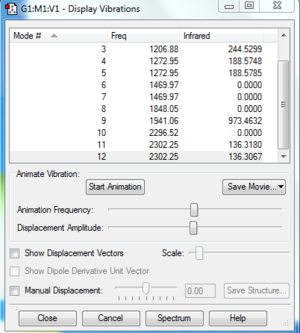
INDEPENDENT WORK
1. How many modes do you expect from the 3N-6 rule?
2. Which modes are degenerate (ie have the same energy)?
3. Which modes are "bending" vibrations and which are "bond stretch" vibrations?
4. Which mode is highly symmetric?
5. One mode is known as the "umbrella" mode, which one is this?
6. How many bands would you expect to see in an experimental spectrum of gaseous PH5?
1. There are 6 atoms in a molecule of PH<sub>5</sub>, so 12 modes are expected. 2. Modes 1 and 2 are degenerate, modes 4 and 5 are degenerate, modes 6 and 7 are degenerate, and modes 11 and 12 are degenerate. 3. Modes 1-7 are 'bending' vibrations while modes 8-12 are 'bond stretch' vibrations. 4. Both the 'bending' mode and the 'bond stretch' mode are symmetric. 5. N/A 6. 5 bands would be expected to be seen.
Charge distribution in PH5
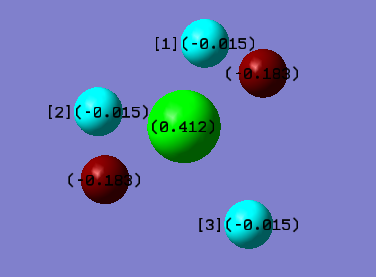
Phosphorous is the most positive because it is less electronegative/more electropositive than hydrogen. Since it has a lower electronegativity, it has a greater charge. The 2 hydrogen atoms that are 180° apart from each other have a more negative charge than the 3 hydrogen atoms that are 120° apart from one another.
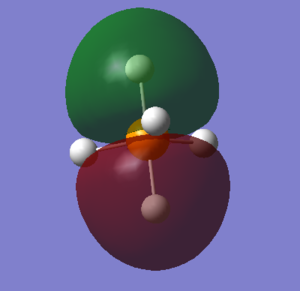
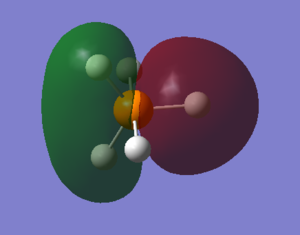
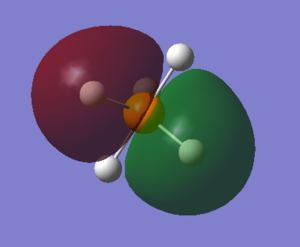
PH5's molecular orbitals
what AOs contribute to the MO? Is the MO bondng, antibonding or a mixture. Is the MO deep in energy, in the HOMO/LUMO region or high in energy? Is the MO occupied or unoccupied? What effect will your MOs have on bonding?
The electronic configuration of P and H are 1s2 2s2 2p6 3s2 3p3 and 1s1 respectively. With phosphorous as the central atom in trigonal bipyramidal structures, generally (and in the case of PH5, the 3s, three 3p and one 3d orbitals hybridise, forming five sp3d hybrid orbitals, according to valence bond theory.
1. LUMO (Lowest Unoccupied Molecular Orbital) : As perhaps given away by the name, this molecular orbital is unoccupied. PH5's LUMO has an energy value of 0.04278 eV, it is the highest of the 5 molecular orbitals chosen, and LUMOs are generally primarily involved with electrophilic reactions. This MO is an antibonding orbital, therefore if it were filled, no bond would be formed. One sp3d orbital (from the phosphorous) and one 1s orbital (from the hydrogen) contribute to the LUMO and its corresponding bonding orbital.
2. HOMO (Highest Occupied Molecular Orbital) : Again, via its name it can be told that this molecular orbital is occupied. PH5's HOMO has an energy value of -0.21463eV and HOMOs are generally involved with nucleophilic reactions. This MO is also antibonding, but despite it being filled, the bond is still formed because the LUMO is not filled. One sp3d orbital and one 1s orbital contribute to the HOMO and its corresponding bonding orbital.
3. The second highest occupied molecular orbital : The energy of this MO is -0.40948 eV. It is relatively high in energy, but lower than that of the LUMO and HOMO. This MO is also antibonding, but despite it being filled (occupied), the bond is still formed because the LUMO is not filled. One sp3d orbital and one 1s orbital contribute to this MO and its corresponding bonding orbital.
4. The third highest occupied molecular orbital : The energy of this MO is -0.42682 eV. It is comparatively high in energy, lower than the LUMO and HOMO but higher than the majority of the other molecular orbitals. This MO is also antibonding, but despite it being filled (occupied), the bond is still formed because the LUMO is not filled. One sp3d orbital and one 1s orbital contribute to this MO and its corresponding bonding orbital.
5. The fourth highest occupied molecular orbital : The energy of this MO is -0.42682 eV. It is moderate in energy; somewhere in the middle. This MO is also antibonding, but despite it being filled (occupied), the bond is still formed because the LUMO is not filled. One sp3d orbital and one 1s orbital contribute to this MO and its corresponding bonding orbital.

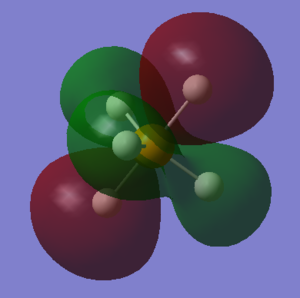
Independent work
1. Answering questions on the vibrational modes of N2, H2 and PH5.